|
#3
9th August 2018, 11:02 AM
| |||
| |||
| Re: Bsc Biotechnology Syllabus Mumbai University
The Syllabus for F. Y. B.Sc.- Biotechnology Program offered by University of Mumbai is as follows: SEMESTER I Basic Chemistry-I USBT 101 Unit I Nomenclature and Classification Nomenclature and Classification of Inorganic Compounds: Oxides, Salts, Acids, Bases, Ionic, Molecular and Coordination Compounds Nomenclature and Classification of Organic Compounds: Alkanes, Alkenes, Alkynes, Cyclic Hydrocarbons, Aromatic Compounds, Alcohols and Ethers, Aldehydes and Ketones, Carboxylic Acids and its derivatives, Amines, Amides, Alkyl Halides and Heterocylic Compounds Unit II Chemical Bonds Chemical Bonds: Ionic Bond: Nature of Ionic Bond, Structure of NaCl, KCl and CsCl, factors influencing the formation of Ionic Bond. Covalent Bond: Nature of Covalent Bond, Structure of CH4, NH3, H2O, Shapes of BeCl2, BF3 Coordinate Bond: Nature of Coordinate Bond Non Covalent Bonds: Van Der Waals forces: dipole - dipole, dipole induced dipole. Hydrogen Bond: Theory of Hydrogen Bonding and Types of Hydrogen Bonding (with examples of RCOOH, ROH, Salicylaldehyde, Amides and Polyamides). Unit III Water and Buffers Chemistry of Water: Properties of Water, Interaction of Water with Solutes (Polar, Non-Polar, Charged), Non-Polar Compounds in Water Change in its Structure and the Hydrophobic Effect, Role of Water in Biomolecular Structure and Function and Water as a Medium for Life Solutions: Normality, Molarity, Molality, Mole fraction, Mole concept, Solubility, Weight ratio, Volume ratio, Weight to Volume ratio, ppb, ppm, millimoles, milliequivalents (Numericals expected). Primary and Secondary Standards: Preparation of Standard Solutions, Principle of Volumetric Analysis Acids and Bases: Lowry-Bronsted and Lewis Concepts. Strong and Weak Acids and Bases - Ionic Product of Water - pH, pKa, pKb Hydrolysis of Salts Buffer solutions Concept of Buffers, Types of Buffers, Derivation of Henderson equation for Acidic and Basic buffers, Buffer action, Buffer capacity(Numericals expected.) pH of Buffer Solution. Syllabus for F. Y. B.Sc.- Biotechnology University of Mumbai  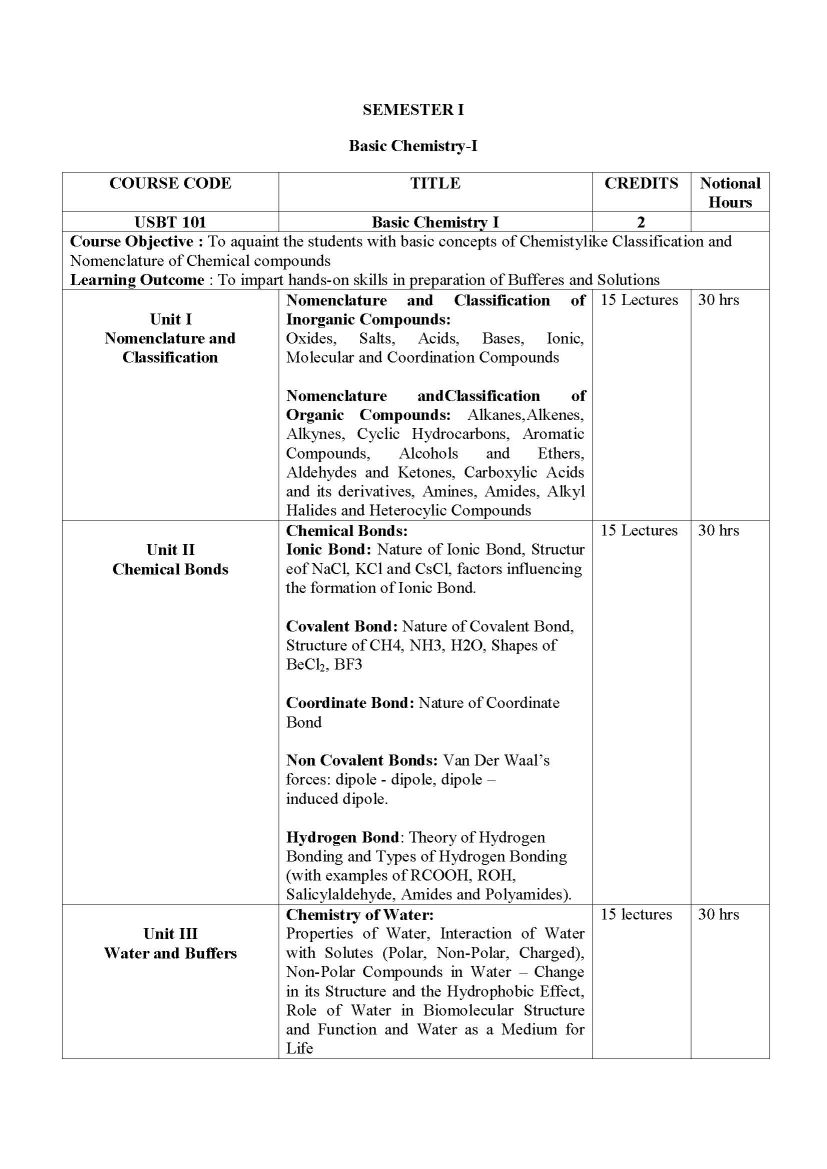 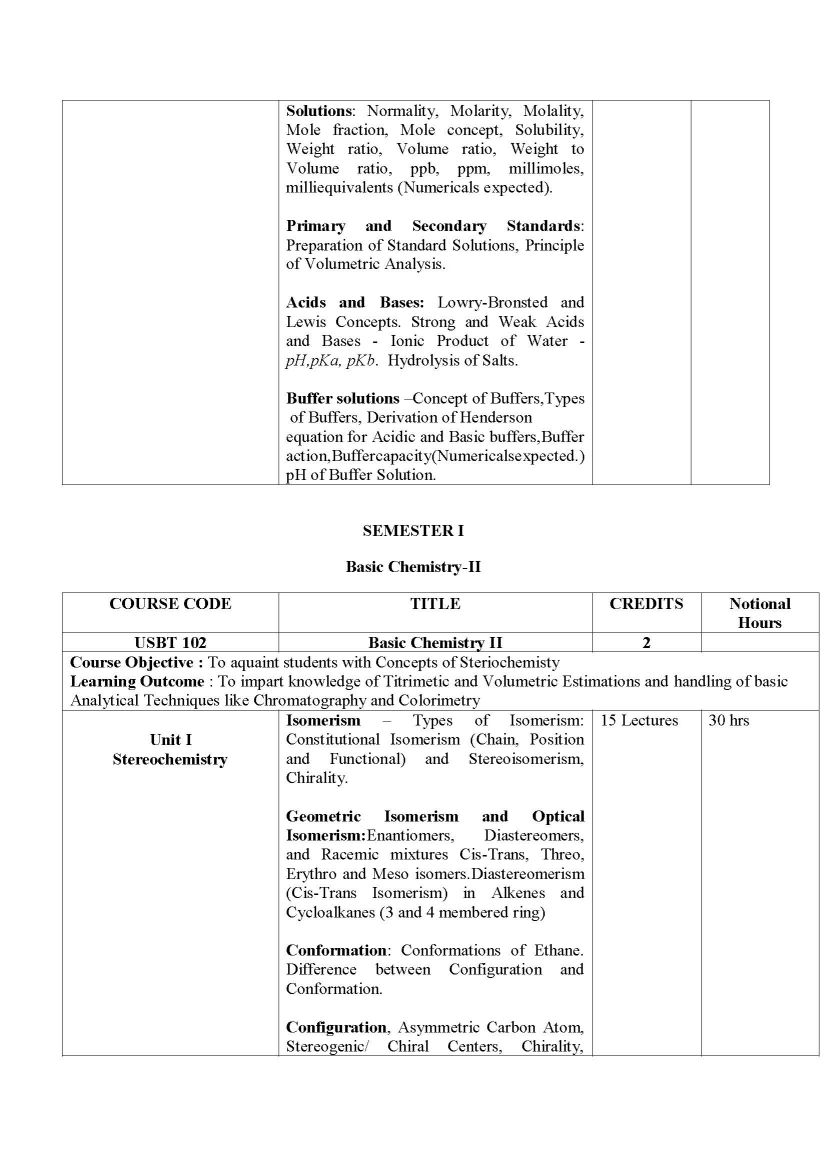 |