|
#2
31st July 2014, 09:06 AM
| |||
| |||
| Re: 12th standard previous years question papers for the Chemistry
This is the CBSE 12th standard previous years question papers for the Chemistry: 29. (a) Give reasons for the following: (i) Bond enthalpy of F2 is lower than that of Cl2. (ii) PH3 has lower boiling point than NH3. (b) Draw the structures of the following molecules: (i) BrF3 (ii) (HPO3)3 OR (a) Account for the following: (i) Helium is used in diving apparatus. (ii) Fluorine does not exhibit positive oxidation state. (iii) Oxygen shows catenation behavior less than sulphur. (b) Draw the structures of the following molecules. (i) XeF2 (ii) H2S2O8 30. (a) Although phenoxide ion has more number of resonating structures than Carboxylate ion, Carboxylic acid is a stronger acid than phenol. Give two reasons. (b) How will you bring about the following conversions? (i) Propanone to propane (ii) Benzoyl chloride to benzaldehyde (iii) Ethanal to but-2-enal     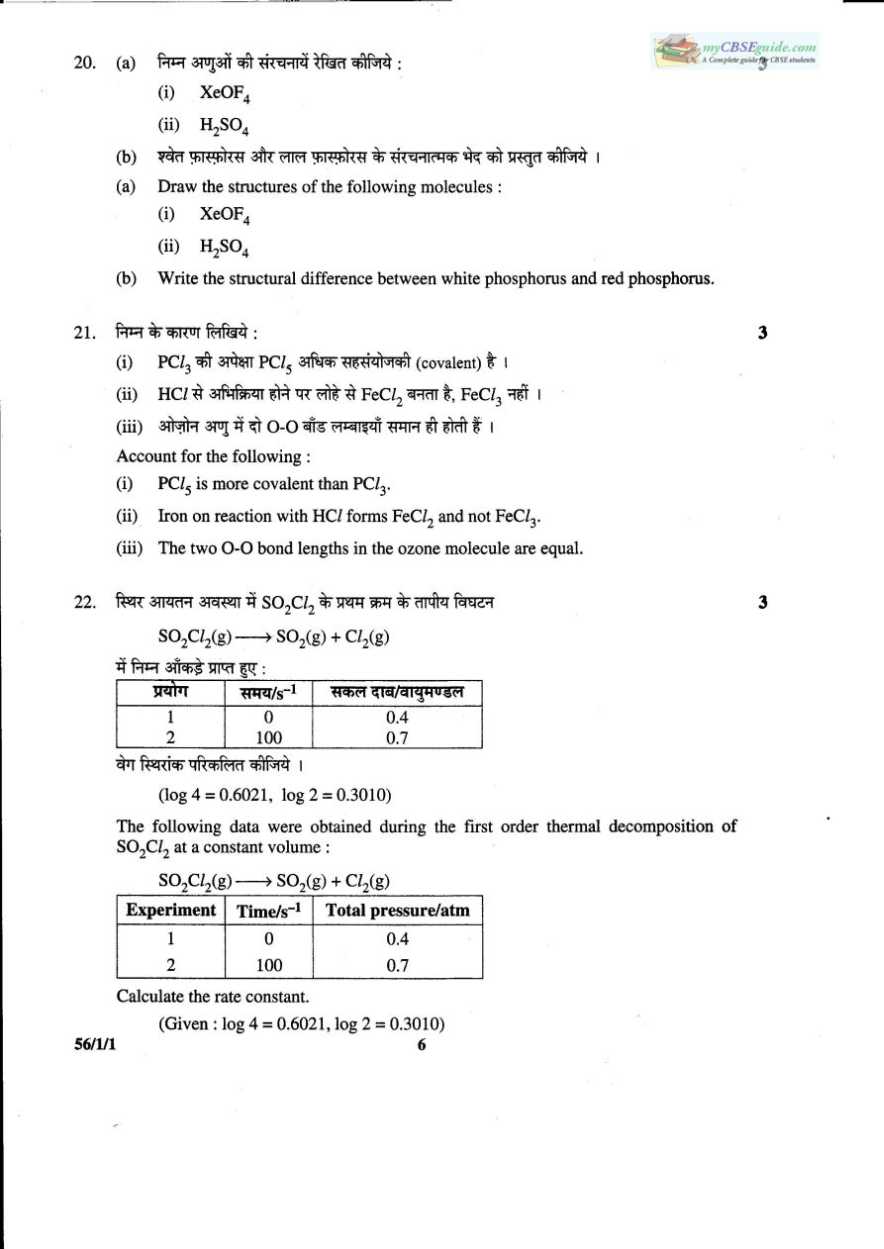 Remaining questions are in the attachment, download it freely from here: |