|
#2
3rd August 2014, 09:05 AM
| |||
| |||
| Re: WBJEE Exam Solved Question Papers
Here I am giving you question paepr for west Bengal Joint entrence examination in a PDF file attached with it . Q. 1 – Q. 45 carry one mark each, for which only one option is correct. Any wrong answer will lead to deduction of 1/3 mark. 1. In diborane, the number of electrons that account for bonding in the bridges is (A) Six (B) Two (C) Eight (D) Four Ans : (D) 3. A van der Waals gas may behave ideally when (A) The volume is very low (B) The temperature is very high (C) The pressure is very low (D) The temperature, pressure and volume all are very high Ans : (C) Hints : A van der waals gas may behave ideally when pressure is very low as compressibility factor (Z) approaches 1. At high temperature Z > 1. 4. The half-life for decay of 14C by β-emission is 5730 years. The fraction of 14C decays, in a sample that is 22,920 years old, would be (A) 1/8 (B) 1/16 (C) 7/8 (D) 15/16 Ans : (D) 5. 2-Methylpropane on monochlorination under photochemical condition give (A) 2-Chloro-2-methylpropane as major product (B) (1:1) Mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane (C) 1-Chloro-2-methylpropane as a major product (D) (1:9) Mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane Ans : (C) 6. For a chemical reaction at 27°C, the activation energy is 600 R. The ratio of the rate constants at 327°C to that of at 27°C will be (A) 2 (B) 40 (C) e (D) e2 Ans : (C)   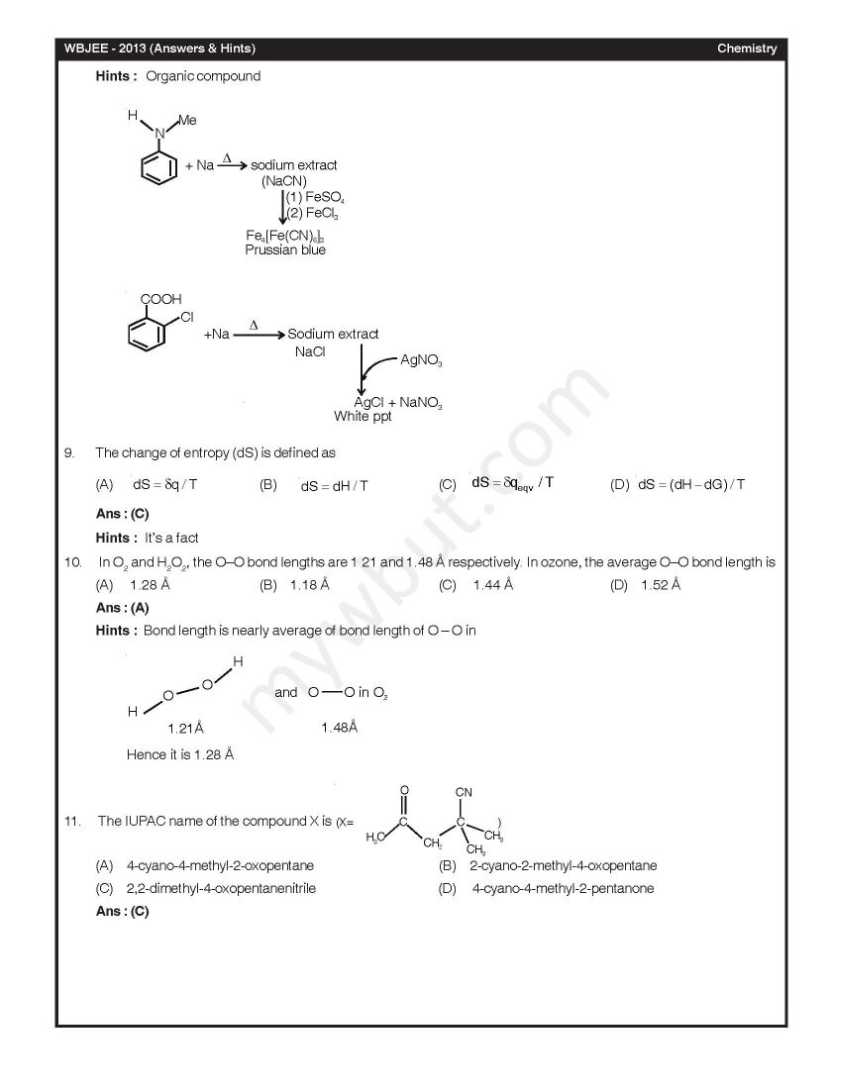  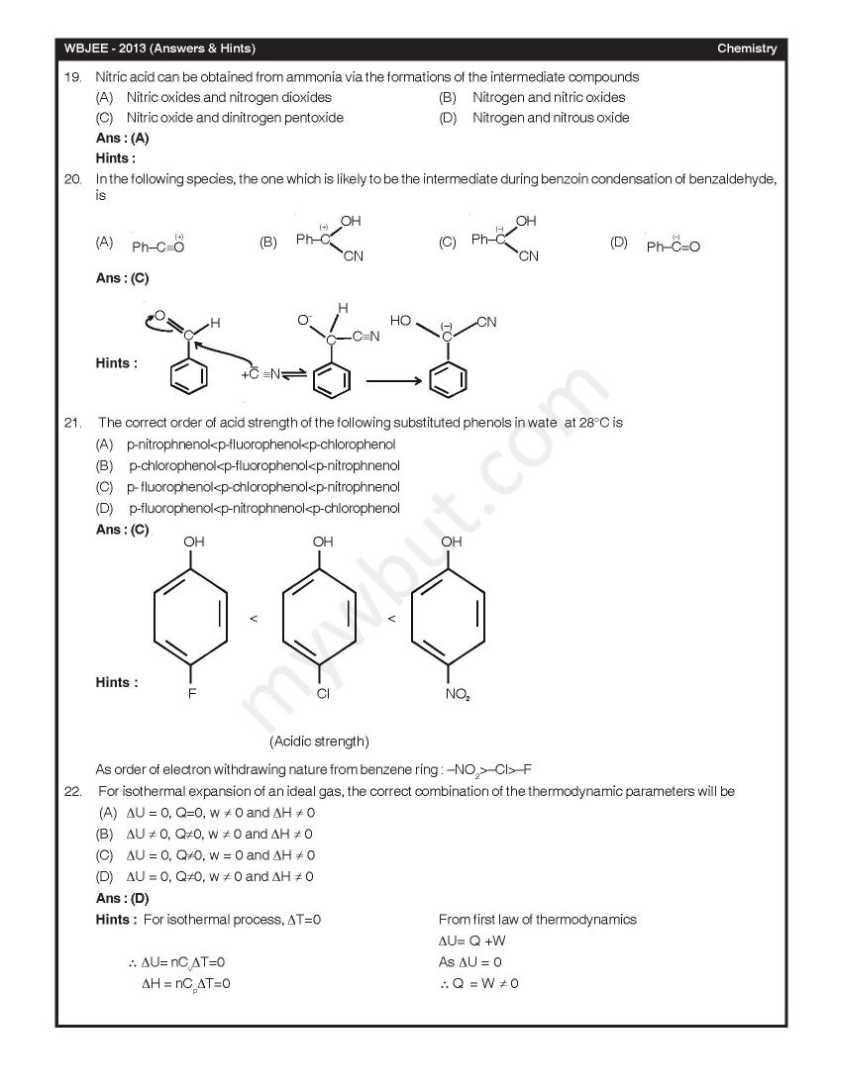 |