|
#2
21st February 2015, 02:19 PM
| |||
| |||
| Re: TIFR Biology Previous Papers
Hello friend as you want the previous year question Papers of TIFR Biology so here I am providing you the same…. 1. A father’s blood group is AB; the mother’s is O. Which of the following blood groups could appear in their offspring? a. AB b. O c. A or B d. None of the above 2. Chandrayaan-1, India’s unmanned probe to the moon was recently in the news because it produced evidence for the presence of a. Liquid water b. Titanium c. Water molecules d. Permafrost 3. In 2009, a survey revealed that more children than average were born in Indian cities that had above-average numbers of Cellphone towers. This could be because: a. Cellphone radiation can influence biological processes. b. More cellphone towers are placed in highly populated areas. c. Fewer single people tend to have mobile phones than married people. d. The number of cellphone towers is an indication of average income. 4. Assume people’s birthdays are uniformly distributed amongst 365 days in a year (ignore the leap years). What is the minimum number of people that you must collect in a room to be sure that at least two share a birthday? a. 23 b. 365 c. 366 d. 1825 5. What proportion of the genes of two worker bees from a hive will be identical? a. 50% b. 25 - 75 % c. 100% d. depends on the parentage of the bees concerned 6. What volume of 20% Sucrose would you use to make 2mL of 5% Sucrose solution a. 0.0005 mL b. 0.05mL c. 0.5mL d. 0.005mL 7. The plot of concentration of product formed in a hypothetical chemical reaction versus time follows an exponential curve. What will be the shape of the plot of the net forward rate of the reaction versus time? a. exponential b. linear c. parabolic d. hyperbolic. 8. What is the effective H3O+ concentration in a 10 mM Phosphate buffer at pH 4.0? a. 10-4 x 10-14 M b. 10-4 + 10-7 M c. 10-4 M d. none of the above 9. You are trying to walk across the slippery floor of a 10 meter wide room. You take uniform steps of 0.5metre each. There is a 10% probability that you will slip and fall at every step. What is the chance that you will make it half-way across, and then slip to fall exactly in the middle of the room? a. (0.1)10 x (0.9) b. (0.1) x (0.9)10 c. 0.5 d. None of the above 10. The heights of members of the staff at an institute follow a nearly normal distribution with mean 160 cm and standard deviation 5 cm. Which of the following is not true? a. Approximately 50% of the members of staff at this institute have heights less than 160 cm; b. Approximately 10% of the members of staff have heights greater than 175 cm; c. Approximately 2.5% of the staff members have heights less than 152 cm; d. The peak of the distribution is around 160 cm. 11. If you hold a nail against a piece of ice, the end in your hand will soon become cold. Why is this? a. Heat flows from the nail to the ice. b. Cold flows from the ice to the nail. c. Answers A and B are equivalent, thus both are correct. d. Heat flows from the nail into your hand, leaving the nail cold. 12. A population of synchronous E. coli cells is allowed to divide and grow at the rate of one binary division every 30 minutes. Suppose there are 4x10^6 cells immediately following a division event. The number of E. coli cells that were present 5 hours previously is approximately: a. 0.24x10^6 b. 1.66x10^5 c. 3.9x10^3 d. 1.024x10^2 13. If log (y) = log (x)m then from the figure shown below, what is the value for ‘m”? a. m = 3 b. m = 1.5 c. m = 1 d. m = 2 14. Which of the following is the most likely graph of the function: y = Ax2 + Bx4? a. Plot B b. Plots A and C c. Plot D d. Plots D and B 15. Which of the following is inconsistent with the standard global warming hypothesis? 1. Certain regions of the earth can experience increased cold spells. 2. Warming is caused by increased levels of greenhouse gases in the atmosphere. 3. Variations in the earth’s orbit are the primary cause of current global warming. 4. Global warming only refers to average global temperatures, not seasonal variations. a. 3 and 4. b. 1 and 2. c. 3 alone d. 1 alone BIOLOGY: 1. Fruitflies have a red eye colour that is determined by the gene RED. Allele R gives a red eye and allele r gives a white eye. R is dominant over r. A single red eye parent and a single white eye parent were mated. Only 50% of the resulting progeny had red eyes. With respect to the RED gene, which of the following best describes the genotype of the red eyed parent? a. rr b. Rr c. RR d. Any of the above 2. The pI of a protein is 8.3. To carry out ion-exchange chromatography of this protein at pH=7.0, the following should be used to ensure binding and fractionation: a. Cation exchanger resin b. Anion exchanger resin c. Reactive ion exchanger resin d. Polyanion exchanger resin 3. Genetic sequence data were used to construct the following evolutionary tree Which of the following is NOT true? a. Mice are descended from the last common ancestor of humans and rats. b. Rats are descended from the last common ancestor of humans and mice c. Humans are descended from the last common ancestor of rats and mice d. Humans, mice, and rats have a common ancestor 4. A significant proportion of students at TIFR seem to have descended from Chengis Khan. This information was inferred from: a. Mitochondrial DNA b. Single Nucleotide Polymorphisms c. Y chromosome analysis d. Ancient DNA 5. The recognition sequence for the restriction endonuclease AstWI is GRCGYC where R is any Purine and Y is any Pyrimidine base. In a truly random DNA sequence what will be the average distance (measured in base pairs) between successive AstWI cut sites? a. 1024 b. 512 c. 4096 d. 24 6. Jitesh carried out a steady-state kinetics experiment for an enzyme degradase with and without molecule X as part of the reaction mixture. The reactions were carried out for 5 minutes and he got the following results: Substrate Concentration (milliMolar) Amount of product formed without X (milliMoles) Amount of product formed with X (milliMoles) 3.5 8.5 8.3 These results suggest that: a. Molecule X is a competitive inhibitor b. Molecule X is an allosteric inhibitor c. Molecule X is a cofactor d. Molecule X has no effect on catalysis 7. Irshath is recording the electrical activity of neurons in a cat when the cat is shown light bars at various angles. Two neurons respond to these light bars in the following manner: What can you infer from the above data? a. Bar angle does not affect neural response in Neuron1 or 2 b. A bar at 50 degrees elicits no response in either of the two neurons c. A bar at 20 degrees elicits 50% of the maximal response in Neuron 2 d. For any given angle the difference in response levels of Neuron1 and 2 is a constant. 8. In the northeastern states of India, Yaks are a common species. Additionally, cows are also present as well as hybrids between Yaks and cows. The following figure represents genetic variation at ‘cow’ specific and ‘Yak’ specific genetic loci. Probability of being a Yak Which one of the following statements is true? a. Most animals are hybrids b. Most animals either Yaks or cows c. There are more cows than Yaks d. All hybrids have the same proportion of Yak versus cow genes in their genome 9. A student conducted an experiment where CO2 and N2 were bubbled through water in beakers A and B respectively. He recorded the pH in each of the solutions every 5 min. What is the most valid conclusion the student could draw from these results: a. The change in pH was too small to be significant b. Bubbling CO2 through water makes it more acidic c. Bubbling N2 through water makes it more acidic d. Both CO2 and N2 make water more acidic. 10. Experiments were carried out on plants living in different environments to measure the size of leaf stomata. Previous investigations have shown that plants transpire more water when the size of stomata is larger. Which of the graphs best represents a plant living in a dry environment? a. A b. B c. C d. D 11. Champa is trying to characterize a gene s4, which has been previously implicated in early development in mice. Her attempts to characterize the phenotype failed because embryos from crossed homozygous mutant parents did not survive. However, she was able to get some viable homozygous embryos when heterozygous parents were crossed. Which of the following can explain this observation? a. s4 is a critical gene for early development and is maternally required* b. s4 is a critical gene for early development and is an imprinted gene c. Both a and b d. None of the above 12. Alzheimers’ protein-A multimerizes at pH 2 to form aggregates which leads to Alzheimers’ disease. In the brain the pH is always neutral ~7. So what leads to aggregation of A that induces Alzheimers’ in people greater than 60 years of age? a. Older brains are more acidic. b. At pH 7, there will be very fast kinetic equilibrium between oligomers and monomers which can change with age to induce aggregation c. At pH 7, the reaction does proceed to oligomerization but the rate is so low that it takes 60 years to form aggregates d. Aggregates form during intermittent drops in pH throughout your life. 13. Mutations in two genes x and y in a diploid organism when present togather cause lethality. Which of the following may be inferred about the nature of the gene product? a. The product of the genes function as an oligomer b. The two genes fall in the same complementation group c. None of above d. The product is a homo-oligomer 14. A gene is transcribed at the maximum rate of k per second, and codes for a transcription factor (TF). This transcription factor inhibits the transcription of its own gene, and this is the only way this gene and TF are regulated. Which of the following graphs is most likely to describe how the steady-state concentration of the TF depends on the value of k? a. A and C b. C c. B d. D 15. Genes X, Y and Z are located on one chromosome. Cross-over frequency between three genes is: Cross over Frequency Gene Pairs 36% X-Z 10% Y-Z 26% X-Y Which one of the following best represents the gene location on the chromosome? a. Z_______________________X_________Y b. Z_________Y_______________________X c. X_________Y_______________________Z d. Y_________X_______________________Z CHEMISTRY: 1. A weak base is present in a beaker and a weak acid is titrated into it. The pH of the solution in the beaker is plotted vs. the volume (in ml) of the weak acid added to the beaker. Which plot below most likely represents the results of this titration? a. Plot B b. Plots C and D c. Plot A d. Plot D 2. The enthalpy of a reaction is plotted vs. its reaction coordinate below. Which of the following is true about the forward reaction? a. The reaction is spontaneous b. The reaction is endothermic c. A catalyst will not be useful for this reaction d. All of the above 3. What type of orbitals are shown in the following picture? a. A bonding σ orbital b. An anti-bonding σ orbital c. A bonding π orbital d. An anti-bonding π orbital 4. At STP, which of the following will have highest no. of molecules a. 8 grams of methane gas b. 16.8 litres of methane gas c. 0.1 moles of methane gas d. 8 grams of oxygen gas 5. The half life of a radioactive element is 72 hours. In how many days will the radioactivity fall to 1/32th of its original value? a. 9 days b. 18 days c. 15 days d. 12 days 6. How many grams of solid NaH2PO4 and Na2HPO4 are required to prepare 1dm3 of 0.1 M phosphate buffer of pH=7.1. pKa2 is 6.8 for buffer. Atomic weight Na=23, P=31, O=16. a. NaH2PO4 =4.00 gram, Na2HPO4= 9.46 gram b. NaH2PO4=9.46 gram, Na2HPO4= 4.00 gram. c. NaH2PO4 =0.4 gram, Na2HPO4= 0.946 gram d. NaH2PO4=0.946 gram, Na2HPO4= 0.4 gram. 7. A molecule contains only nitrogen and oxygen. 30.40% of this molecule is nitrogen (by mass). If the molar mass of the molecule is 92 g/mol, what is the molecular formula? a. NO2 b. N2O2 c. N2O4 d. NO 8. The molecular weight of ethanol (C2H5OH) is 46 and its density is ~0.8 g/cm2. What would be the molar concentration of ethanol in a 23% (v/v) wine? a. 4 molar b. 0.5 molar c. 2 molar d. None of the above. 9. The equilibrium constant (K) for a reaction A + B = AB is 1 x 10-6 M, where K is defined as K = [A][B]/[AB]. If the rate constant for association of A and B is 2 x 107 M-1 S-1, what is the rate constant (RC) for the dissociation of AB and the average life-time (T) of the complex? a. RC= 2 x 10-13 S-1 and T = 1 microsecond b. RC = 20 S-1 and T = 50 milliseconds c. RC = 5 S-1 and T = 200 milliseconds d. RC = 2 x 10-7 S-1 and T = 0.05 microseconds 10. An SN2 reaction of an asymmetric carbon of a compound always gives a. An enantiomer of the substrate b. A product of opposite optical rotation c. A mixture of diastereomers d. A single stereoisomer 11. An unknown, containing some combination of alanine, lysine or aspartic acid, is subjected to paper electrophoresis at pH = 7.0. Ninhydrin treatment (to stain them) shows some amino acid at the negative electrode and some amino acids have not moved from the center. No amino acid is found at the positive electrode. Which amino acid(s) is (are) in the unknown? a. Alanine and lysine but not aspartic acid b. Alanine and aspartic acid but not lysine c. Only alanine d. All the three amino acids 12. Srikanth wants to characterize the enzymatic properties of a novel enzyme (nE1) involved in NAD salvage pathway in human cells. It turns out that this pathway and the enzyme are evolutionarily conserved. But previous studies indicate that this pathway is critical in human cells. Manohar aims to establish that nE1 is the most important enzyme in this pathway. During his studies he happened to compare nE1 activities from mice (m-nE1) and humans (h-nE1). Interestingly, his experiments showed that the Km values for m-nE1 and h- nE1 were 10mM and 20mM, respectively. Based on these results what is the best interpretation? a. nE1 catalyzes the rate limiting step in mice but not in humans b. m-nE1 has more affinity to the substrate than h-nE1 c. nE1 catalyzes the rate limiting step in humans but not in mice d. h-nE1 has more affinity to the substrate than m-nE1 13. Equal weights of methane and hydrogen are mixed in an empty containerat 250C. The fraction of the total pressure exerted by hydrogen is a. ½ b. 8/9 c. 1/9 d. 17/19 14. Match the following substances with the models i. SeO2 ii. Be Cl2 iii. PBr3 iv. BCl3 a. i = I, ii = II, iii = III, iv = IV b. i = II, ii = I, iii = III, iv = IV c. i = I, ii = II, iii = IV , iv = III d. i = II, ii = I, iii = IV, iv = III 15. Which of the following resonating structures of l-methoxy -1, 3-butadiene is least stable? PHYSICS: 1. When you make ice cubes, the entropy of the water a. does not change b. Increases c. decreases d. may either increase or decrease, depending on the specific process used 2. A big elephant and a small stone are dropped from an extremely tall building. Which of the following statements is true? (ignore wind and turbulence) a. The elephant experiences more air resistance and its terminal velocity is less than the stone b. The elephant experiences less air resistance and its terminal velocity is more than the stone c. The elephant experiences more air resistance and its terminal velocity is more than the stone d. The elephant experiences less air resistance and its terminal velocity is less than the stone 3. Where is the centre of mass of the atmosphere of the Earth located? a. close to the surface of the Earth b. close to the centre of the Earth c. close to the top of the atmosphere d. halfway between the surface of the Earth and the top of the atmosphere 4. Suppose that a bird lands on a bare 14,000 V power line. What is likely to happen? a. It will be killed by current flow b. It will not be hurt because it has a high body resistance. c. It will not be hurt because there is negligible potential difference across its body d. None of the above is true. 5. What is the net stiffness, measured between the points A and B, of the following network of springs? Each spring has a stiffness of 1 N/m. a. 1/4 N/m b. 3/5 N/m c. 5/3 N/m d. 5/2 N/m 6. Two masses, m and M, are connected to a pulley system attached to a table, as in the diagram. What is the minimum value for the coefficient of static friction between mass M and the table if the pulley system does not move? a. m/M b. M/m c. g (m/M) d. g (M/m) 7. An object of mass 3 kg is hung from a spring of spring constant 50 N/m. How far is the equilibrium position of this spring system from the point where the spring exerts no force on the object? a. 0.15 m b. 0.3 m c. 0.5 m d. 0.6 m 8. Objects cannot move with a speed faster than the speed of light. This implies an upper limit on their: a. Momentum b. kinetic energy c. both momentum and kinetic energy d. Neither 9. A stone is tied to a thread of constant length and rotated in a horizontal plane at constant angular velocity. Which of the following quantities changes with time? a. Kinetic energy of the stone b. Potential energy of the stone c. Momentum of the stone d. Total energy of the stone 10. You have been asked to crystallize the sugar in sugar syrup. What is the quickest way to do this? a. Heat the syrup in a pressure cooker b. Heat the syrup in an evacuated chamber connected to a vacuum pump c. Heat the syrup in an open pan at low flame d. All the above methods are equally quick 11. The Centre of Mass and Centre of Gravity of an object can be different if the object is a. a cylinder b. an irregularly shaped body c. a sphere d. None of the above 12. An ideal Voltmeter (V), an ideal ammeter (A), two 100 Ohm resistances and a 5V battery are connected as shown in the figure. The voltmeter and ammeter readings respectively will be a. 0.5 Volts, 0.2 amperes b. 5.0 Volts, 0.0 amperes c. 1 Volt, 25 milliamperes d. 5 Volts, 50 milliamperes 13. A monochromatic beam of red light is incident at an angle of 60 degrees on the face of a prism. It passes through the prism and light emerging from the opposite face of the prism is observed. Which of the following is true? a. Because of dispersion, the emerging beam will be white in color b. The incident light beam will not bend in the prism c. The incident light beam will bend in the prism, and will disperse into different colors d. The incident light beam will bend in the prism, but will not disperse into different colors 14. If the earth was perfectly spherical and nothing obstructed your vision, as in the diagram below, how far away would the horizon be? a. 1 and 2km b. between 4 and 5km c. between 12 and 13km d. between 23 and 24 km 15. There are precisely 3 primary colours of light: red, green, and blue. a. This is because of the physics of light. b. This is because of the chemistry of paint. c. This is because of the biology of the human eye. d. None of the above.  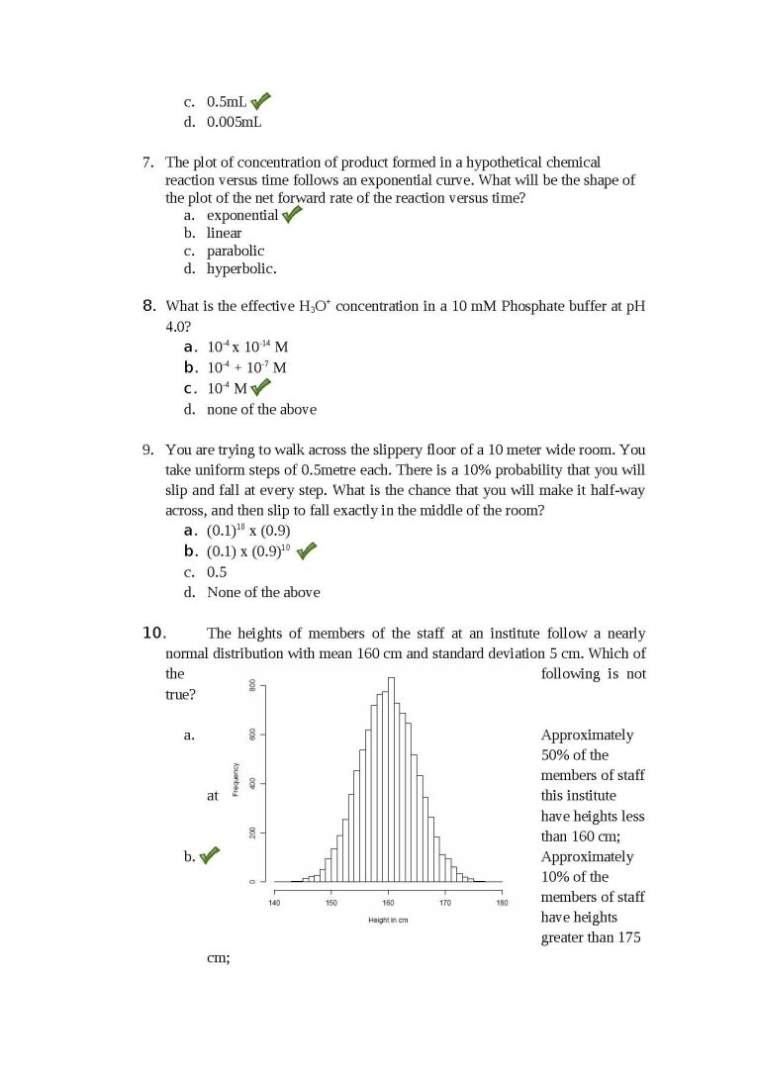  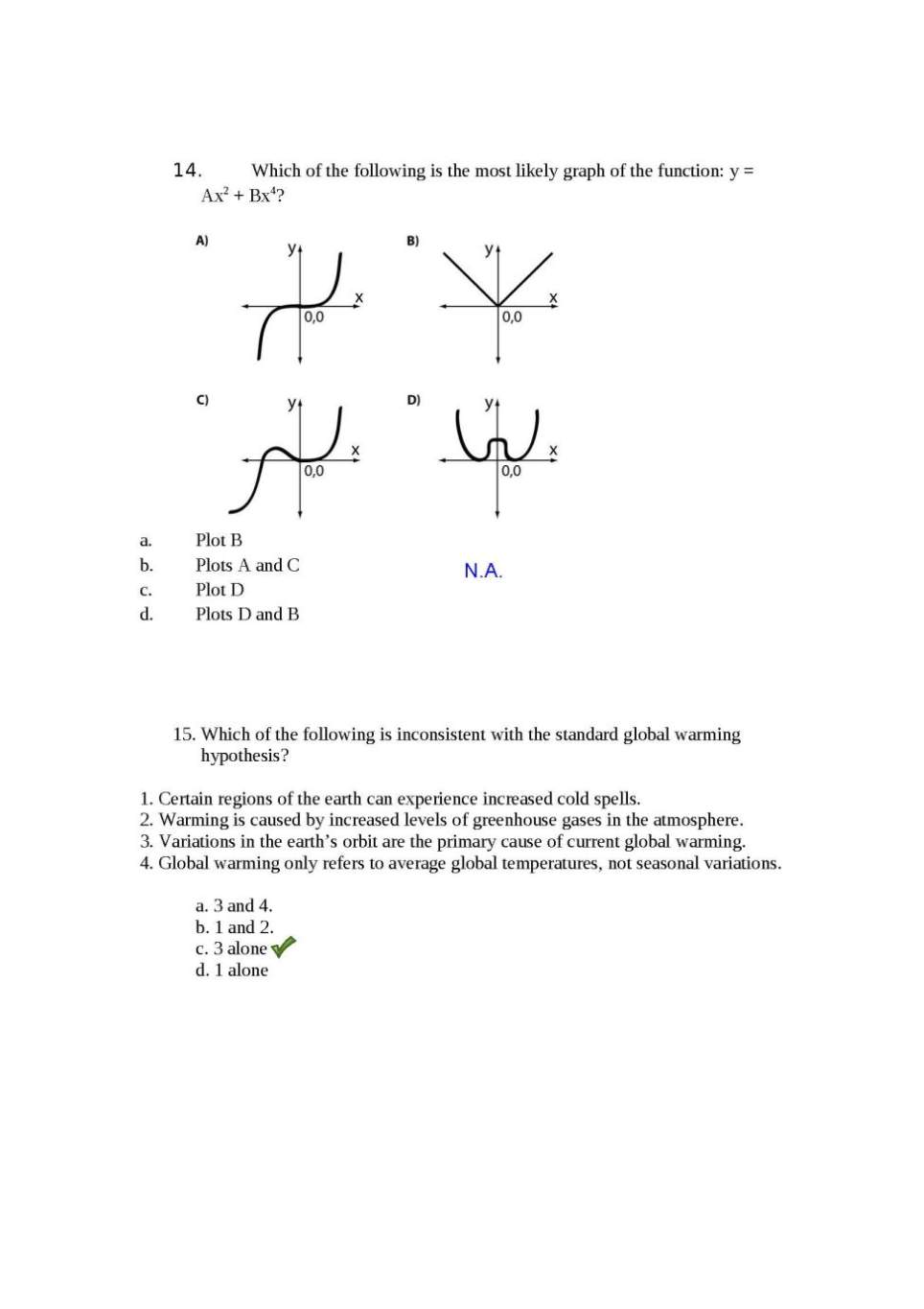 Rest of the paper here I am attaching pdf file please download it.. |