| Re: Kakatiya University Pg Chemistry Syllabus
As you want to download syllabus of 2nd Semester of M.SC Chemistry Course of Kakatiya University, so here I am providing detailed syllabus for your reference:
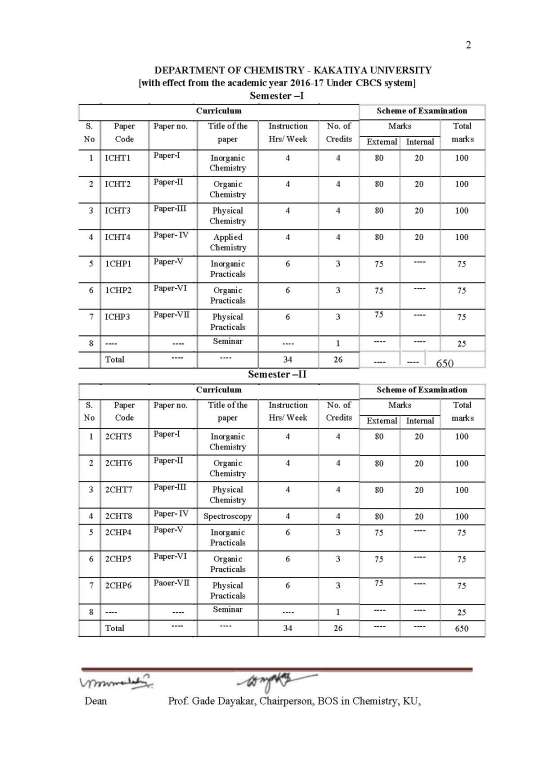
Kakatiya University M.Sc Chemistry 2nd Semester Syllabus:
2CHT5 Paper-I Inorganic Chemistry
2CHT6 Paper-II Organic Chemistry
2CHT7 Paper-III Physical Chemistry
2CHT8 Paper- IV Spectroscopy
2CHP4 Paper-V Inorganic Practicals
2CHP5 Paper-VI Organic Practicals
2CHP6 Paper-VII Physical Practicals
Seminar
PAPER-I: INORGANIC CHEMISTRY (2CHT5)
Electronic-spectra of metal complexes:
Free-ion terms and energy levels - Electron configuration, Microstates and Terms. Calculation of microstates for p and d configurations, Russel-Saunders (L-S) coupling.
Organometallic Compounds:
Classification and nomenclature of organometallic compounds, Principles of synthesis of organometallic compounds. Synthesis, structure and properties of organometallic compounds of Al and Sn. 18-electron rule and stability of organotransition metal compounds. Synthesis, structure and bonding of olefin, allyl and cyclopentadienyl organometallic compounds of Fe, Pd and Pt. Applications of organometallic compounds of B and Si in organic synthesis. Organometallic compounds in homogeneous catalysis Hydrogenation, Hydroformylation and Isomerization processes.
Bioinorganic Chemistry:
Metal ions in biological systems - Brief survey of metal ions in biological systems, Basic principles underlying biological selection of elements, Physiological effects of metal ion concentration.
Oxygen transport and storage - Haemoglobin and Myoglobin, Geometric, electronic and magnetic aspects of dioxygen binding, oxygen adsorption isotherms and cooperativity, Physiological significance of hemoglobin, Role of globin chain in haemoglobin.
Metals/ Metal compounds in medicine - Introduction, Metal deficiency and disease, Iron deficiency, Zinc deficiency, and Copper deficiency; Metals used for diagnosis and radiodiagnosis; Lithium, Gold and Platinum compounds used in therapy.
Ligational aspects of diatomic molecules:
Metal Carbonyls: Classification of metal carbonyls, General methods of preparing metal carbonyls, Ligational properties of Carbon monoxide (CO), Donor and acceptor molecular orbitals of CO, Bonding modes of CO, Evidence for multiple bonding, Eighteen electron rule, Electron counting methods i) Neutral atom method and ii) Oxidation state method, Structural
Metal nitrosyls: General methods of preparing metal nitrosyls, Donor and acceptor molecular orbitals of nitric oxide (NO), Bonding modes of NO.
PAPER-II: ORGANIC CHEMISTRY (2CHT6)
Named reactions in organic synthesis:
Beckmann rearrangement, Mannich reaction, Michael addition, Dienone-Phenol rearrangement, Robison annulation, Favorski reaction, Baylis-Hillman reaction, Shapiro reaction, Ugi reaction, Grubbs reaction, Heck reaction, Suzuki coupling, Stille coupling, Sonogashira coupling, and Buchwald reaction.
Stereochemistry II:
Conformational analyses of Cycloalkanes: Conformations of small and medium sized rings and conformations of mono and disubstituted cyclohexanes. Factors governing the reactivity of equatorial and axial substituents attached to the cyclohexane ring Relative stability and reactivity of conformational diastereomers Stereochemistry of bicyclic systems involving five and six numbered rings. Conformations of cyclohexanone - Stereochemistry of addition to the carbonyl group in rigid cyclohexanone system. Use of physical methods (dipole moment, IR and NMR) in determining the preferred conformers of simple organic molecules such as 1,2-dihalo ethanes, halohydrins and vicinal diols.
ORD studies: Optical rotation and optical rotatory dispersion, axial haloketone rule, octant rule, applications of ORD studies in the determination of configuration and conformation of organic molecules.
Protection of functional groups and Nucleic acids:
Protection of functional groups: Principles of (1) protection of alcohols Ether formation: methyl, benzyl, allyl, methoxy ethoxy methyl (MEM), THP, silyl, and TBDMS ethers; Ester formation methyl, benzoyl, tosyl, and p-nitro benzoyl ester (2) protection of diols acetal, ketal and carbamate formation (3) protection of carboxylic acids Ester formation: methyl, benzyl, t-butyl, p-nitrobenzyl, p-bromophenacyl, and silyl esters (4) protection of amines Amide and Carbamate formation with formyl, acetylation, benzoyl, benzyloxy carbonyl (CBZ), tert-butyloxycarbonyl (BOC), tert-butyl azido formyl, phthaloyl, di-tert-butyl pyrocarbonyl, Fluorenylmethyloxycarbonyl (FMOC), and triphenyl methyl groups (5) protection of carbonyl groups acetal, ketal, 1,3-dioxolane, 1,3-dioxane, 1,3-dithiolane, 1,3-oxathiolane and 1,3-dithiane formation. Nucleic acids: Isolation, structure, and properties of RNA & DNA synthesis of nucleosides, nucleotides, and synthesis of polynucleotides. Biosynthesis of RNA and DNA.
Nonbenzenoid aromatic compounds:
Concept of aromaticity, Robinsons sextet theory, Huckels rule, basis for the Huckels rule, limitations of the Huckel’s rule- Alternant and Non-alternant hydrocarbons Craigs rule Various Nonbenzenoid aromatic molecules Synthesis and properties of aromatic 3,4,5,6,7,8-membered rings, metallocenes, annulenes, heteroannulenes, azulenes, fullerenes(C60), Sydnones Antiaromatic compounds,
PAPER-III: PHYSICAL CHEMISTRY (2CHT7)
Thermodynamics II:
Statistical Thermodynamics: Thermodynamic probability of distinguishable and indistinguishable particles-most probable distributionentropy and probability (BoltzmannPlanck equation), MaxwellBoltzmann distribution lawpartition function and types. Translational, rotational, vibrational and electronic functionsRelation between thermodynamic functions (E, H, S and G) and partition functions-factorization into translational, rotational, vibrational and electronic contributions of monoatomic and diatomic molecules. Sackur-tetrode equation of entropy. Equilibrium constant. Quantum Statistics: Basic concepts of quantum statisticsBoseEinstein and FermiDirac statisticscomparison with MaxwellBoltzmann statistics.
Solid State:
Bonding in metals: Valence bond theory of metallic bond, Free electron TheoryMolecular orbital approach to the Band theory of solidsclassification of solids Insulators, conductors, and semiconductorstypes of semiconductors, temperature effect on conductivity, photoconductivity and photovoltaic effectp and n junctions. Defects in crystals: Point defects, colour centers, line defects and plane defects. Superconductivity: Superconductivity and types of superconductors Theories of superconductivity BCS theory Applications of superconductors. High temperature superconductors - Structure of defect perovskites. High superconductivity in cuprates. Specific heats of solids: Dulong and Pettit's law, Einstein theory and Debye theory of specific heats. Solid state reactions: Classification and theory of solid state reactions DWagner’s\ theory - examples.
Chemical Kinetics - II:
Effect of substituent on the rate of reaction Hammett’s and Taft’s equations use of s and r constants and extended Hammett equation. YukawaTsuno equationNonlinear Hammett’s PlotsIsokinetic temperature and its determination. Acid-base catalysis: Homogeneous acidbase catalysismechanism of acid-base catalysis-protolytic and prototropic mechanism.
Enzyme catalysis: Specific action and classification of enzymesKinetics and mechanism of single substrate reactionMichaelisMenten Kinetics. Production detection and estimation of free radicals.
Download complete syllabus by this attachment………….
|