|
#2
5th August 2015, 08:18 AM
| |||
| |||
| Re: Burdwan University Department of Chemistry
As you want to get the list of courses offered in Burdwan University Department of Chemistry so here is the information of the same for you: University of Burdwan was established back in the year of 1960 and it is a public university located in Bardhaman, West Bengal, India. It was established by the West Bengal government as a teaching and affiliating university on 15 June 1960 with six graduate departments and thirty undergraduate colleges spread over three districts that come under the jurisdiction of the university. Courses Offered: B.Sc Chemistry M.Sc Chemistry M.Phil Chemistry List of Department of Chemistry Affiliated Colleges: Asansol Girls' College Asansol Asansol - 713304, Burdwan INDIA Phone : (91)-0341-2254098/2256442 Banwarilal Bhalotia College Asansol - 713303, Burdwan INDIA Phone : (91)-0341-2202842, 0341-2274968 Burdwan Raj College Post - Burdwan Burdwan INDIA Phone : (91)-0342-2567787/2565841/2557843 Durgapur Government College Durgapur Durgapur - 713214, Burdwan INDIA Phone : (91)-0343-2500534, 0343-2546068 Email : durgapurgovtcollege@yahoo.com Gushkara Mahavidyalaya P.O. Guskara Gushkara - 713128, Burdwan INDIA Phone : (91)-03452-255006, 03452-255105 Kalna College Kalna - 713409, Burdwan INDIA Phone : (91)-03454-2255032 Email : kalnacollege@gmail.com , kalnacollege@sancharnet.in Katwa College Katwa - 713130, Burdwan INDIA Phone : (91)-03453-255049/255050 Email : katclgbu@sancharnet.in M.U.C. Women's College Burdwan - 713104, Burdwan INDIA Phone : (91)-0342-2533168 Shyamsundar College Shyamsundar - 713424, Burdwan INDIA Phone : (91)-03451-260226/ 260016 Triveni Debi Bhalotia College Raniganj - 713347, Burdwan INDIA Phone : (91)-0341-2444780, 0341-2444275 Email : principal_tdbc@indiatimes.com Vivekananda Mahavidyalaya, Burdwan Burdwan - 713103, Burdwan INDIA Phone : (91)-0342-2541208, 0342-2541521 Email : vm1964@rediffmail.com, vivekanandamahavidyalaya@yahoo.co.in Raniganj Girls' College Raniganj - 713347, Burdwan INDIA Phone : (91)-0341-2257107 Dr. Bhupendra Nath Dutta Smriti Mahavidyalaya Hatgobindapur - 713145, Burdwan INDIA Phone : (91)-0342-2584960/ 2584616 Bankura Christian College Bankura - 722101, Bankura INDIA Phone : (91)-03242-250924/26632257(R) Ramananda College Bishnupur - 722122, Bankura INDIA Phone : (91)-03244-252059 Saldiha College Saldiha - 722136, Bankura INDIA Phone : (91)-03242-262224/252286(R)/0341-26 Abhedananda Mahavidyalaya Post - Sainthia Birbhum INDIA Phone : (91)-03462-262292/263449/255623 Panchmura Mahavidyalaya Panchmura - 722156, Bankura INDIA Phone : (91)-03243-268227 Bolpur College Bolpur - 731204, Birbhum INDIA Phone : (91)-03463-252290(O)/252201(R) Krishna Chandra College Hetampur - 731124, Birbhum INDIA Phone : (91)-03462-244236 (O) / 244201(R) Rampurhat College Rampurhat - 731224, Birbhum INDIA Phone : (91)-03461-255018 Suri Vidyasagar College Suri - 731101, Birbhum INDIA Phone : (91)-03462-255504/256965(R) Aghore Kamini Prakash Chandra Mahavidyalaya Bengai - 712611, Hooghly INDIA Phone : (91)-03211-246235 Bejoy Narayan Mahavidyalaya Itachuna - 712147, Hooghly INDIA Phone : (91)-03213-272275 Chandernagore Government College Chandernagore - 712136, Hooghly INDIA Phone : (91)-033-26835290 Hooghly Mohsin College Chinsurah - 712101, Hooghly INDIA Phone : (91)-033-26802252 Netaji Mahavidyalaya Post - Arambag Hooghly INDIA Phone : (91)-03211-255102/2255012 Raja Rammohan Roy Mahavidyalaya Radhanagore, Nagulpara - 712406, Hooghly INDIA Phone : (91)-03211-266221 Email : rrrm@vsnl.net Rabindra Mahavidyalaya Champadanga - 712401, Hooghly INDIA Phone : (91)-03212-255104 Sreegopal Banerjee College Bagati Magra - 712148, Hooghly INDIA Phone : (91)-033-26842706 Email : sgbc_2003@yahoo.com Durgapur Women's College Durgapur - 713209, Burdwan INDIA Phone : (91)-0343-2562852 Bidhan Chandra College Asansol - 713304, Burdwan INDIA Phone : (91)- 0341-2202520, 0341-228320 Sree Ramkrishna Sarada Vidya Mahapith Kamarpukur - 712612, Hooghly INDIA Phone : (91)-03211-244224 Deshbandhu Mahavidyalaya Post - Chitaranjan Chittaranjan - 713331, Burdwan INDIA Phone : (91)-0341-2525449 Bankura Sammilani College Kenduadihi Bankura - 722102, Bankura Phone : (91)-03242-250741 Email : bscollegebankura@sancharnet.in, bscollegebankura@yahoo.co.in Hooghly Women's College Pipulpati - 712103, Hooghly INDIA Phone : (91)-033-26802335 Sonamukhi College Sonamukhi - 722207, Bankura INDIA Phone : (91)-03244-275251/263463(R) Khalisani Mahavidyalaya P.O. Khalisani Chandernagore - 712138, Hooghly INDIA Phone : (91)-033-26829517/26825530 Email : khalisanicollege@gmail.com Sarat Centenary College Dhaniakhali - 712302, Hooghly INDIA Phone : (91)-03213-255282 Email : principal_sccollegednk@rediffmail.com Here for your reference I am giving you the syllabus of B.Sc Chemistry of University of Burdwan: Some content of the file has been given here: Part I Theoretical Paper I Inorganic 1. Atomic structure and periodic properties (14 lectures) Bohr’s model, Sommerfeld’s extension, de Broglie’s wave particle duality; Heisenberg’s uncertainty principle and Schrödinger’s equation (qualitative); significance of ψ and ψ2; radial density, angular probability, characteristics of s-/p-/d-orbital, Aufbau principle, Pauli’s exclusion/antisymmetry principle (statement and implication), Hund’s rules, Slater’s rules, quantum defect Mendeleev-Seaborg’s periodic table: basis and possible extension; periodic properties: atomic radius, ionic radius, covalent radius, van der Waals radius, ionization energy, electron affinity, electronegativity and its different scales, orbital/group electronegativity, ionic potential, diagonal relationship, work function; aperiodicity 2. Bonding and structure (14 lectures) Different bonds: ionic, covalent, dative, retrodative, hydrogen, metallic, σ-/π-/µ-/δ-, banana (3c-2e); different weak forces, varied hybrid (sp, ds, sp2, sp3, d3s, dsp2, sp3d, d2sp3, d3sp3 etc) orbitals, hypervalence, resonance, bond polarity, dipole moment, Fajan’s rules; VB, LCAO, MO (qualitative idea on homo- /heteronuclear di-/tri-/polyatomic molecules such as AX2 to AX6), symmetry, energy and overlap, HOMOLUMO, VB-MO comparison; bond multiplicity, bond strength and related implications Prediction of structures and shapes of molecules: Helferich rules, VSEPR theory, steric number, Bent’s rule; non-rigid molecules, Berry pseudorotation 3. Acid-base and donor-acceptor (8 lectures) Different concepts, Pauling’s rules, solvent acidity/basicity, Drago-Wayland equation, donor/acceptor number, Gutmann’s rules, Hammett acidity function, super acid, solid acid, surface acidity, factors affecting acidity/basicity, HSAB principle, symbiosis, HOMO-LUMO and acid-base interaction; basis, measurement and anisotropicity of hardness/softness, pictorial diagram of frontier orbitals 4. Redox system (6 lectures) Complementary/non-complementary redox reactions, standard/formal electrode potentials; influence of pH, complex formation and precipitation reaction on formal potential; Latimer/Forst/Pourbaix diagram, electrochemical series and its implication towards metal extraction principle, basis of redox titration, redox indicator, disproportionation, comproportionation 5. Coordination chemistry I (18 lectures) Tassaert’s observation, Jorgensen’s proposition, Werner’s theory; Lewis dot structure, classification and binding modes of ligands: classical, non-classical (π-complexing), σ-/(σ + π)-donor, σ-donor + π-acceptor, bridging (EO/EE) and bridging loop, chelator (cis/trans) and chelate effect, congregator, innermetallic, ambidentate, sequestering, flexidentate, innocent, non-innocent, tripod, macrocycle, podand, coronand, crown ether, cryptand, metalloorganic, organometallic, cyclometallated, Schiff-base, metalloligand and duplex behaviour Synthesis of compounds of different nuclearities; internal parameters: metal and ligand; external parameters: temperature, pressure, solvent, reagent, counter ion, aerobic/anaerobic; stabilization of different oxidation states, choice of starting materials, self assembly Types of isomerism, statistical numbering system, enumeration of isomers; factors effecting isomer population, interplay of steric and electronic factors, isomorphism and doping, structural equilibria, resolution of optical isomers; IUPAC nomenclature Paper II: Organic: 1. General introduction (4 lectures) Nomenclature of organic molecules with special reference to polycyclic, bridge head, aromatic, heteroaromatic and heterocycles compounds, molecular weight (preliminary idea about mass spectroscopy), molecular formula 2. Structure and properties (8 lectures) Nature of bonding in aliphatic, alicyclic, aromatic and heterocyclic compounds; bond length, bond strength, bond angle and their variations in compounds with sp3, sp2 and sp hybridized carbon atoms; orbital pictures of methane, ethane, ethene, ethyne, allene and benzyne; delocalised bonds, resonance, steric inhibition of resonance, hyperconjugation, tautomerism, aromaticity, Huckel’s rules, aromatic, nonaromatic and antiaromatic compounds, non-benzenoid aromatic compounds, Huckel’s rule Inductive and field effects; dipole moment, H-bonding and its effect on physical and chemical properties of organic molecules 3. Introduction to organic reactions (7 lectures) Homolysis and heterolysis of bonds; types of reactions: ionic, radical and pericyclic; Bronsted-Lowry concept, Lewis concept, strengths of acids and bases; effect of solvent on acidity and basicity; relationship between structure and acidity and basicity, acid-base reactions Thermodynamics and kinetics of organic reactions, energy profiles for one-step and two- step reactions, catalyzed reactions, Hammond postulate, principle of microscopic reversibility, kinetically and thermodynamically controlled reactions; methods of determination of organic reactions: study of intermediates, kinetic and stereochemical studies, non-kinetic and kinetic studies with isotopes (primary and secondary kinetic isotope effects), crossover experiments 4. Reactive intermediates (8 lectures) Formation, structure, stability and reactions of classical and non-classical carbocations, carbanions, free radicals, arynes, ylides, carbenes and nitrenes 5. Stereochemistry (20 lectures) Concept of constitution, stereochemical representation: Fischer, Newman, Sawhorse, Flying-wedge and their interconversions, molecular symmetry: plane, centre, simple and alternating axes; symmetry operations, chirality and chiral centre, configuration, configurational nomenclature: D/L, R/S, erythro/threo; optical activity and optical isomerism, optical rotation: specific and molecular; optical purity, enantiomeric excess; resolution of optical isomers; chiral axis in allenes, biphenyls and spiranes and their R/S descriptors Geometrical isomerism (diastereomerism) of molecules with C=C, C=N (oximes) and simple cyclic molecules; cis/trans and E/Z nomenclature Conformation of alkanes and cycloalkanes: dihedral angle and angle of torsion, gauche, eclipsed and staggered arrangements; synperiplanar, synclinal, anticlinal, antiperiplanar conformations; conformation-energy diagram of ethane, propane and n-butane, relative stability of conformers on the basis of steric effect, dipoledipole interaction, H-bonding; conformational analysis of cyclohexane and its mono- and di-substituted derivatives with chair, boat and twist boat forms and their symmetry properties and optical activity; strains in molecules: angle, bond, torsional and steric; steric and stereoelectronic factors 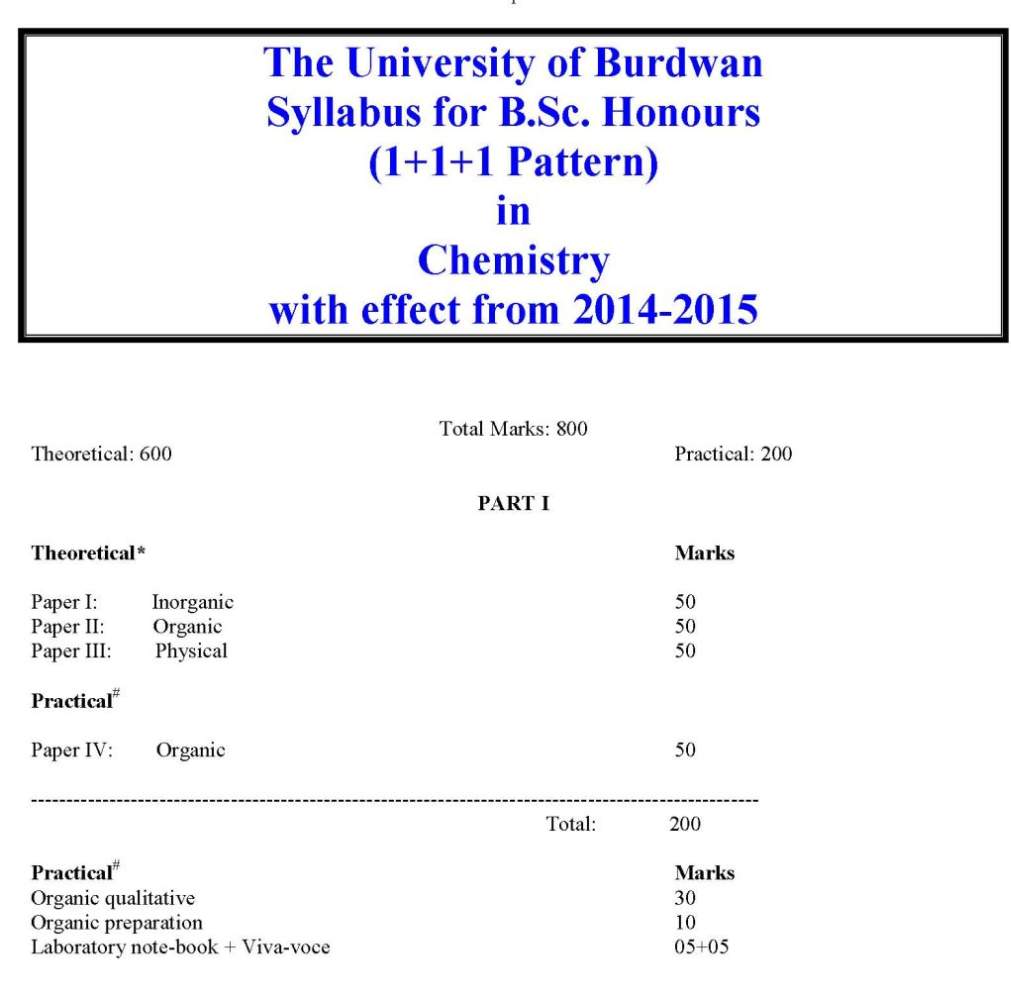 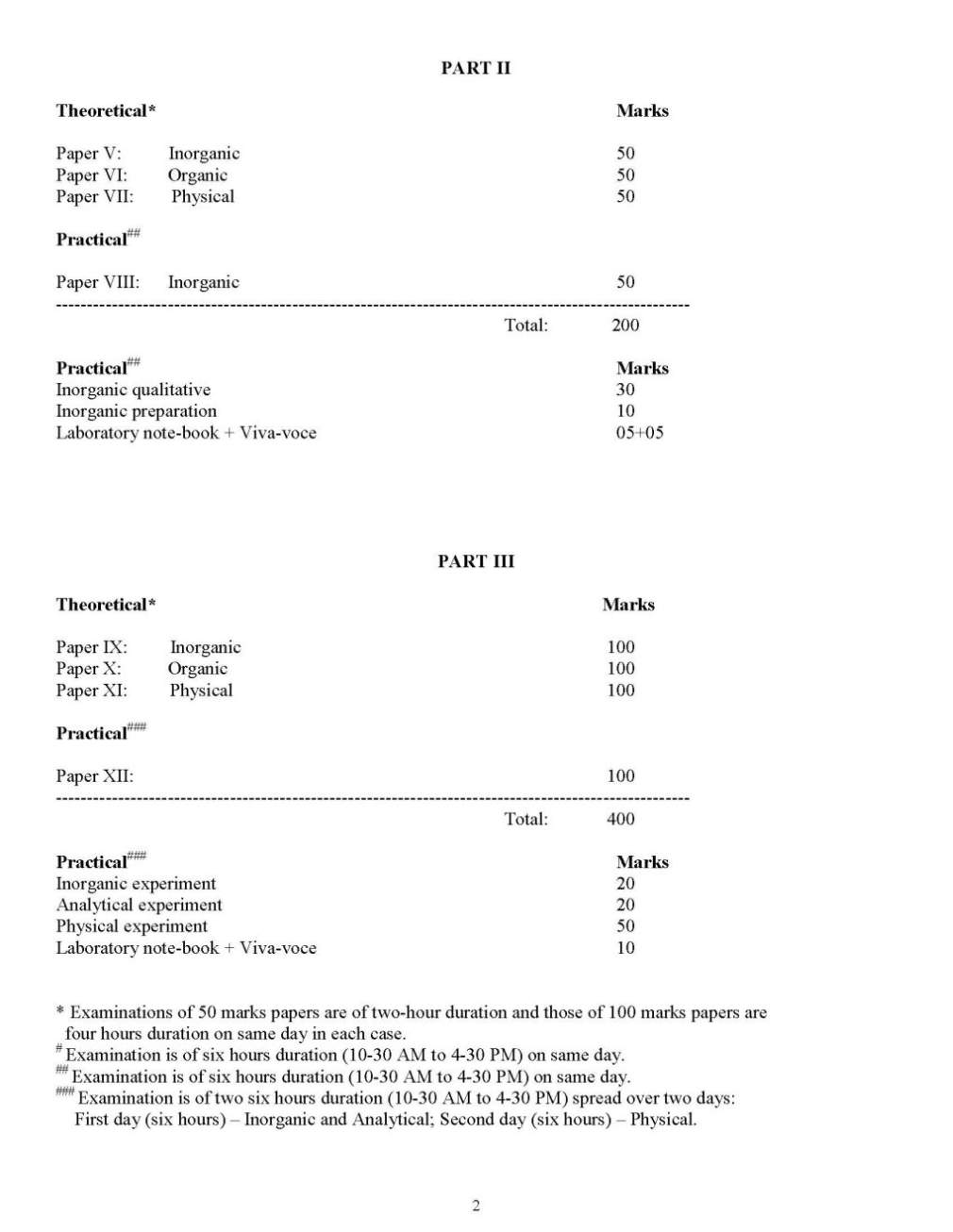    For more detailed information I am uploading a PDF file which is free to download: Contact Details: The University of Burdwan Rajbati Burdwan, West Bengal 713104 India Map Location: [MAP]The University of Burdwan Burdwan[/MAP] |