Here I am sharing the previous year question paper of M.Sc Chemistry entrance exam of Banaras Hindu University
1. The change in entropy when two moles of a monatomic perfect gas is compressed to
half its volume and simultaneously heated to twice its initial temperature is
(1) Rln2 (2) 3Rln2 (3) 5Rln2 (4) 7Rln2
2. A heat engine operates between 1000 K and 600 K. The heat discharged into the cold
sink in a reversible process when 5 kJ of heat is supplied by the hot source, is
(i) 2 kJ (2) 2•5 kJ (3) 3 kJ (4) 5•5 kJ
3. For which of the following processes q = 0, w = 0, AU = 0 and AH = O?
(I) Reversible isothermal process in a perfect gas
(2) Reversible adiabatic process in a perfect gas
(3) Adiabatic expansion of a perfect gas into vacuum
(4) Reversible constant-volume process in a perfect gas
4. The fugacity or a certain gas at 200 K and 50 bars is 25 bars. The difference of its
chemical potential from that of a perfect gas in the same s~te is
(I) 200R In 25 (2) 200Rln2 (3) -200Rln2 (4) -200Rln25
5. The entropy of mixing I mole of )lexane with I mole of )leptane at 298 K is 11•4 JK-1
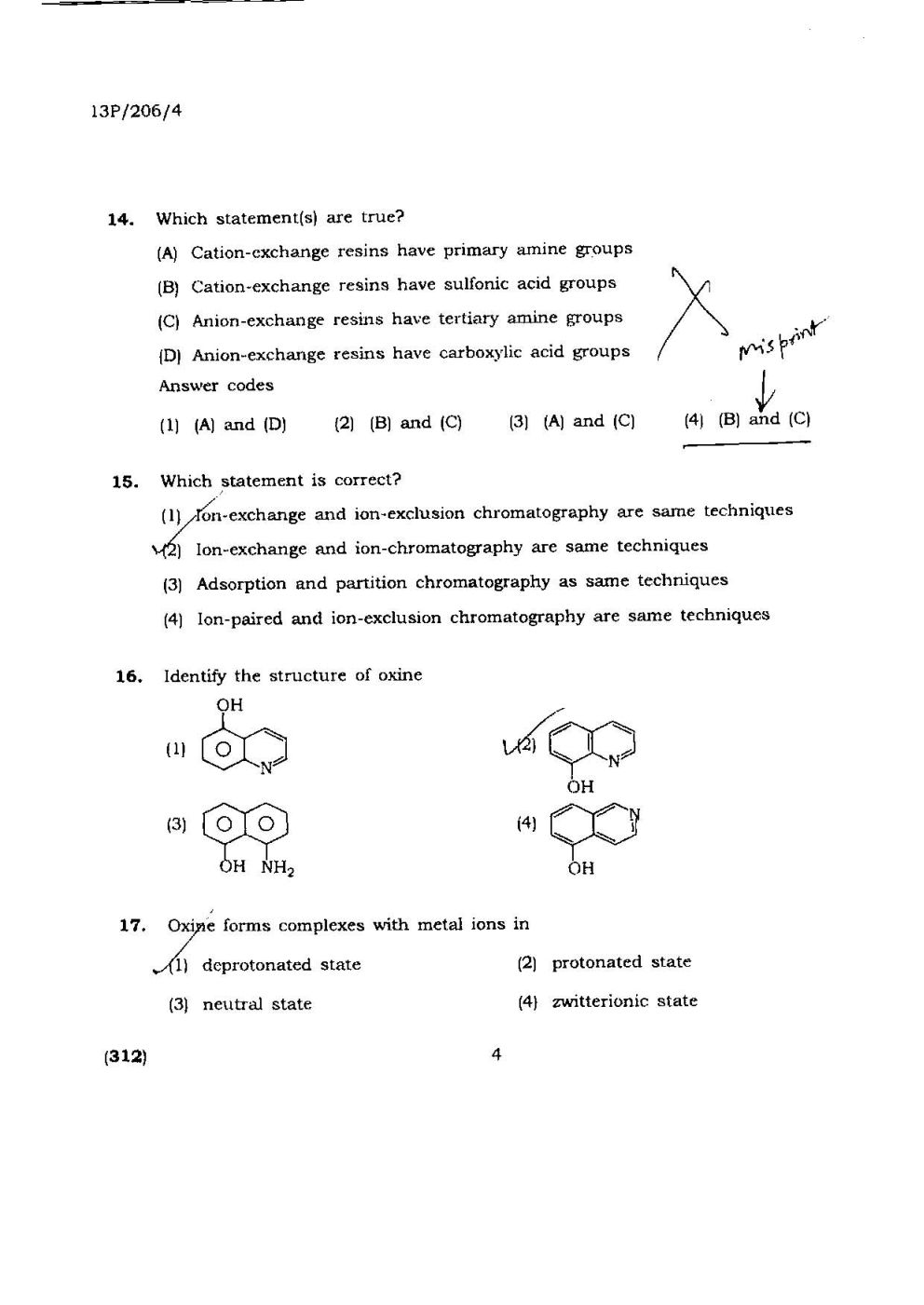
The Gibbs energy 9f mixing (assuming the solution to be ideal) is
(I) -1•72kJ (2) -3•44 kJ (3) 1•72 kJ (4) 3•44 kJ
6. The number of degrees of freedom of the system
KCI03 (s)-= 2KCI(s) +302 (g)
is
(1) zero (2) one (3) two (4) three
7. The expression that relates the partial molar properties of the components in a mixture
is mown as
(1) van\ Hoff equation
(3) Du)lem-Margules equation
(2) Gibbs-Du)lem equation
(4) Raoult's law
8. For a very dilute electrolyte solution with r + < 1, y + should increase with increase in
(273)
(I) solvent's density
(3) ionic strength
(2) solvent's dielectric constant
(4) cationic c)large
9. Which of the following statements is not' correct?
(1) AGmix at constant temperature and pressure must be negative
(2' Intermolecular interactions are negligible in an ideal solution
(3) Solute-solute interactions are negligible in an ideally dilute solution
(4' Activity coefficients are never negative
10. Which of the following statements is correct?
(1) If ~Go> 0, no amount of products can be obtained when the reaction is run at
constant temperature and pressure
(2) It is possible for the entropy of a closed system to decrease substantially in an
irreversible process
(3) In any closed system with P - V work only, G is always minimised at equilibrium
(4) A (TS) =TAS+S'oT
11. The condition for the attainment of phase-equilibrium in a closed electrochemical
system is the equality of
(\) surface potentials
(3) chemical potentials
(2) electric potentials
(4) electrochemical potentials
12. The direction of a chemical reaction at constant temperature and pressure is the
direction of
(273)
(\) decrease of Gibbs free energy of the system
(2) decrease of Helmholtz free energy of the system
(3) increase of entropy of the system
(4) decrease of enthalpy of the system
13. The quantum yield of the photochemical decomposition of HI
HI+hv-> H+I
H+HI-> H2 +1
I+I+M~ 12 +M
with respect to HI is
(1) 0'5 (2) 1 (3) 1'5 (4) 2
14. HI is absorbed strongly on gold. Assuming Langmuir isotherm to apply, the order at the
reaction 2H1~ H2 +12 on gold is
(I) zero (2) 0'25 (3) 0•5 (4) 1
15. Which of the following statements is not correct for Langmuir isotherm?
(1) It applies to monolayer adsorption
(2) Under conditions a « 1 and a (Freundlich exponent) = I, it reduces to Framdlich
isotherm
(3) It applies to dissociative adsorption
(4) It applies to chemisorption
16. The activation energy and entropy of a bimolecular gas phase reaction at 600 K are
200kJmorl and -200JK-1 mol-1 respectively. The free energy of activatiuo is
(1) 70 kJ mol-I (2) 80 kJ mol-I (3) 310 kJ mol-I (4) 320 kJ mol-I
17. Among the following statements which is the correct one?
(273)
(1) The heat of chemisorption is always larger than that of physisorption
(2) Langmuir isotherm specifically assumes the existence of active centres
(3) Promoters are themselves catalysts
(4) Increase in surface tension with concentration leads to negative adsorption
18. The activation energy of the gas-phase association between F2 and 1Fs. a first-order
reaction in each of the reactants, is 58•6 kJ mol-I, The activation enthalpy at 340 K is
(1) 53 kJ moI-1 (2) 55•8kJmol-1 (3) 58•6 kJ mol-1 (4) 61.4kJmor1
19. In a photochemical reaction A -+ 2B +C, the quantum efficiency with 500 nm light is
2 x 102 mol einstein -1. After exposure of 300 m moles of A to the light, 2 m moles of B is
formed. The number of photons absorbed by A is
(1) lxl018 (2) 3x 1018 (3) 6xl018 (4) 9x 1018
20. The condition for which the reaction rate of an enzymolysis that follows
Michaelis-Menten kinetics, is half its maximum value, is
(1) (8)« K M
21. For the me_chanism
(2) (8)~KM (3) (8)~K M 12
A2 ~ 2A (fast)
A +B -> P (slow)
the reaction order with respect to A2 is
(I) 0 (2) 0•5 (3) 1
(4) (8)>> K M
(4) 2
22. Which of the following relations does not hold for the activity (A) of a radioactive
substance?
(I) ~ =G)"'''' (2) ~=exp(_0.693_t_)
Ao to.5
(3) to.5/to.1 =ln2 (4) ~=1-0.693..!.... at t .... O
Ao to.5
23. A powder diffraction photograph from tungsten shows lines which indices as (110).
(200), (211), (220), (310), (222), (321), (400), .... The symmetry of the unit cell is
(1) primitive (2) end-centred (3) face-centred (4) body-centred
24. Among the following halides which one fonns van der Walls crystals?
(1) NaCI (2) BeC12 (3) HgCl2 (4) HF
25. A form of CaCO, (c) has orthorhombic lattice with a=5•0 A, b =8•0 A, c=5•6 A and'
density = 3•0 gm em -3 at room temperature. The number of Ca 2+ ions per unit cell of"
the crystal is
(1) 1 (2) 2 (3) 4 (4) 8
26. The ratio of the translational partition functions of D2 and H2 at the same temperature
and volume is
(1) 2 (2) 1-414 (3) 2•83 (4) 4
27. In which of the following systems is the energy level separation the largest?
(1) an electron in a radical in a field of 0•300 T
(2) a 14N nucleus in 600 MHz NMR spectrometer
(3) a proton in the same spectrometer
(4) a deuteron in the same spectrometer
28. Which of the following functions is not an eigenfunction of d
2
2?
dx
(1) cos lex (2) exp(_lex2 ) (3) lex (4) exp(ilex)
29. The ratio of mean molar masses of a given polymer sample as determined by light
scattering, sedimentation and osmotic pressure measurement methods is
(1) 1:1:2 (2) 2:1:2 (3) 1:2:1 (4) 2:2:1
30. In milk at 37°C Lactobacillus acidophilus has a generation time of 75 minutes. What is
the population relative to the initial value at 150 minutes?
(1) 4•0 (2) 2•25 (3) 2•0 (4) 1-75
31. If the pressure of a gas at constant temperature is doubled, the viscosity of the gas will
be
(1) quadrupled (2) doubled (3) halved (4) unchanged
32. Among the following molecules which one shows pure rotation spectra?
(1) N, (2) H2S' (3) CO2 (4) CH.
33. The SI unit of radiation dose is
(1) becquerel (2) curie (3) rad (4) gray
34. It is found tha~ a particle in a one-dimensional box of length of L can be excited to n = 2
state from the ground state by the light of frequency v. If the box length i. doubled, the
frequency needed to produce the above transition becomes
(1) v/4 (2) v/2 (3) 2v (4) 4v
35. For a hydrogen •atom in an n == 4 state, the maximum possible z-component of orbital
angular momentum is
(I) 2h (2) 3h (3) ..ff2 h (4) ,f6 h
36. Which one of the following is an acceptable approximate wave function Cor a state of the
helium atom?
(273)
(1) [Is(l) 15 (2)-1s(1) 1s (2)lIa (I)P(2»
(2) [15 (1)15 (2)lIa (l)a(2)+p(I)P(2))
(3) [1s(I)2s (2)+25 (I) 1s (2)J[a (I)a (2))
(4) [Is (1) 25 (2) +2s (1) Is (2))[a (I) P(2) -p (I) a (2))
37. Which one of the following statements concerning H~ is incorrect?
(1) The non-degenerate LCAO-MOs (without spin) must be either symmetric or antisymmetric
for inversion
{2} The lowest energy MO (without spin} of the molecule is anti-symmetric for
inversion
(3) The ground state has a multiplicity of two
(4) The MOs transform into ACs of the helium ion as the two nuclei are fused together
38. Which one of the following is the correct formula for the lowest energy eigenfunction for
a particle in a one-dimensional box having infmite barriers at x = -L {2 and L (2?
(1) ~ ain(7)
(3) if exp C;

39. Which of the following equations is used to calculate the number of theoretical plates?
(1) tR -to
to
(3) k2
k,
40. Which of the following techniques is based on selectively inducing radioactivity and
measuring the emitted radiation?
(1) Isotope dilution analysis (2) Radiometric titration
(3) Neutron activatiim analysis (4) All of the above
41. Which of the following techniques can be used only for volatile compounds?
(1) Gas chromatography
(3) Ion chromatography
(2) HPLC
(4) All of the above
42. Which of the following refers to ion exchange capacity?
(1) Nature of exchanging ions
(2) Nature of strong cation exchanger
(3) Nature of strong anion exchanger
(4) Total number of ion active groups per unit length of material
43. In electrogravimetry of cations the working electrode is
(I) anode (2) cathode
(3) both cathode and anode (4) neither anode nor cathode
44. Which of the following techniques is/are feasible approach in the detennination of a
substance that cannot be isolated in pure form for gravimetry or for detennination by
other methods?
(1) Neutron activation analysis (2) Isotope dilution analysis
(3) Radiometric titration (4) All of the above
45. Which of the following frequencies corresponds to carbonyl stretch vibration in acids?
(I) 1625 cm-1 (2) 1715 cm-1 (3) 1745cm-1 (4) 1800• em-I
46. A particular vibration in a polyatomic molecule is IR active if during vibration. there is
a change in
(I) poIarizability
(273)
(2) dipole moment (3) frequency
(4) potential energy
47. Moisture in a drug can be determined by
(I) Malaprade reagent (2) EDTA reagent
(3) Karl Fischer reagent (4) chloramine-T reagent
48. Which one is more toxic?
(I) Hg (2) (CH3bHg (3) Hg2+ (4) Hg~+
49. The most efficient technique for the separation of amino acids is
(I) adsorption chromatography
(3) ion-exchange chromatography
SO. Which one is not a pollutant?
(I) CO (2) CO2
51. Which one is the aink of CO2?
(I) Plant (2) Ocean
(2) partition chromatography
(4) paper chromatography
(3) 503 (4) N02
(3) Air (4) Soil
52. Ozone layer is a protective shield against
(I) visible light (2) ultraviolet light
(3) infrared rays
53. Nessler's reagent is
(I) KHgI.
(3) K 2HgI.
(273) 10
(4) cosmic rays
(2) K 2HgI. + NH.OH
(4) KHgI. +NH.OH
For complete paper here is the PDF file