|
#2
26th July 2014, 10:37 AM
| |||
| |||
| Re: Form for M Tech in GGSIPU
Here I am giving you procedure to fill form for M tech admission in Guru Gobind Singh Indraprastha University, Delhi (India) ===go on official website of Guru Gobind Singh Indraprastha University, Delhi ==than click on admission notice 2014 option given on right side of home page of it == Online Application Form for CET option on that page ==now click on apply online option on that page ==so you will get online application form for CET exam ==you have to fill all required details and click on next option so you can easily submit form for it 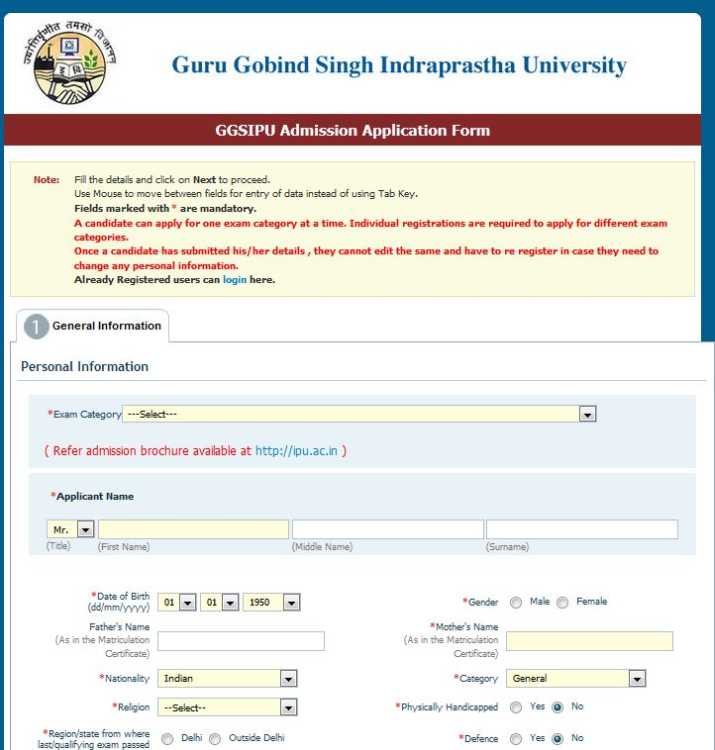 syllabus : Syllabus for CET for M.Tech ( Engineering Physics ) Programme Physics Interference : Young’s double slit experiment, Fresnel’s biprism, Thin films, Newton’s rings, Michelson’s interferometer, Fabry Perot interferometer. Diffraction : Fresnel Diffraction: Zone plate, circular aperture, opaque circular disc, narrow slit, Fraunhofer diffraction : Single slit, double slit, diffraction grating, resolving power and dispersive power. Polarization : Types of polarization, Brewsters law, Malu’s Law, Nicol prism, double refraction, quarter-wave and half-wave plates, optical activity, specific rotation. Lasers : Introduction, coherence, population inversion, basic principle and operation of a laser, Einstein A and B coefficients, type of lasers, He-Ne laser, Ruby laser, semiconductor laser, holography-theory and applications Fibre Optics : Types of optical fibres and their characteristics, ( Attenuation and dispersion step index and graded index fibres, principle of fibre optic communication-total internal reflection, numerical aperture, fibre optical communication network ( qualitative )-its advantages. Theory of Relativity : Galenlian transformations, the postulates of the special theory of relativity, Lorentz transformations, time dilation, length contraction, velocity addition, mass energy equivalence. Thermodynamics : The first law and other basic concepts: dimensions, units, work, heat, energy, the first law of thermodynamics, enthalpy, equilibrium, phase rule, heat capacity, PVT behavior of pure substances, ideal gas, real gas, heat effects. The second law and Entropy : statements, heat engines, Kelvin-Planck and Clausious statements and their equality, reversible and irreversible processes, Carnot cycle, thermodynamic temperature scale, entropy, ent ropy calculations, T-S diagrams, properties of pure substances, use of steam tables and Mollier diagram. Refrigeration and liquefaction : the Carnot refrigerator, the vapor–compression cycle, comparison of refrigeration cycles, liquefaction processes, heat pump. Rankine power cycle. Quantum Mechanics : Wave particle duality, deBroglie waves, evidences for the wave nature of matter – the experiment of Davisson and Germer, electron diffraction, physical interpretation of the wave function and its properties, the wave packet, the uncertainty principle. The Schrodinger wave equation ( 1 – dimensional ), Eigen values and Eigen functions, expectation values, simple Eigen value problems – solutions of the Schrodinger’s equations for the free particle, the infinite well, the finite well, tunneling effect, simple harmonic oscillator ( qualitative ), zero point energy. Quantum Statistics : The statistical distributions, Maxwell Boltzmann, Bose-Einstein and Fermi-Dirac statistics, their comparisons, Fermions and Bosons. Applications: Molecular speed and energies in an ideal gas. The Black-body spectrum and failure of classical statistics to give the correct explanation – the application of Bose-Einstein statistics to the Black-body radiation spectrum, Fermi-Dirac distribution to free electron theory, electron specific heats, Fermi energy and average energy – its significance. Band theory of solids : Origin of energy bands in solids, Kronig-Penny model, Brillouin zones, effective mass, Metals, semiconductors and insulators and their energy band structure. Extrinsic and intrinsic semiconductors, p-n junction diodes- its characteristics, tunnel diode, zener diode, photodiode, LED, photovoltaic cell, Hall effect in semiconductors, transistor characteristics ( common base, common emitter, common collector ). Digital techniques and their applications ( registers, counters, comparators and similar circuits ) A/D and D/A converters. Superconductivity : ZFC and FC, Meissner effect, Type I and II superconductors, the Josephson effect, flux quantization, Cooper pairs, BCS theory, properties and applications of superconductors. X-rays : production and properties, crystalline and amorphous solids, Bragg’s law, applications. Electricity and magnetism : Electric fields, Gauss’ Law, its integral and differential form, applications. Lorentz force, fields due to moving charges, the magnetic field, Ampere’s law, motion of a charged particle in an electric and magnetic field, magnetic and electrostatic focussing, Hall effect, determination of e/m by cathode ray tube, positive rays, Thomson’s parabolic method, Isotopes, Mass spectrographs ( Aston and Bainbridge ), Electron microscope, Cyclotron and Betatron. Overview of Electro – Magnetism : Maxwell’s Equations: The equation of continuity for Time – Varying fields, Inconsistency in ampere’s law Maxwell’s Equations, conditions at a Boundary Surface, Introduction to EM wave. Nuclear Physics : Introduction of nucleus, Nucleus radius and density, Nuclear forces, Nuclear reactions, Cross section, Q-value and threshold energy of nuclear reactions, Basic Idea for Nuclear Reactor, Breeder reactor, The Geiger-Mullar ( G.M. ) Counter, Introduction of Accelerators and its Applications. Numerical Techniques : Interpolations, differentiation, integration; Nonlinear equations, the bisection methods, Newton’s method, root finding; Differential equations, Euler’s method, the Runge-Kutta method; Matrices-inverting, finding eigenvalues and eigenfunctions. Mathematics Linear Independence and dependence of vectors, Systems of linear equations – consistency and inconsistency, rank of a matrix, Gauss elimination method, , Eigen values and Eigen vectors. Successive differentiation, Leibnitz’s theorem, Lagrange’s Theorem, Cauchy Mean value theorems, Taylor’s theorem, Asymptotes, Curvature, Reduction Formulae of trigonometric functions, Properties of definite Integral, Applications to length, area, volume, surface of revolution. Partial derivatives, Method of Lagrange’s multipliers. Jacobeans of coordinates transformations. Double and Triple integrals. Method of separation of variables, homogeneous, linear equations, exactness and integrating factors, linear equations of higher order with constant coefficients, Operator method to find particular integral. Scalar and vector fields, Directional Derivative, Gradient of scalar field, divergence and curl of a vector field. Green’s theorem, Divergence theorem and Stoke’s theorem. Probability : Definition of Sample Space, Event, Event Space, Conditional Probability, Additive and Multiplicative law of Probability, Baye’s Law theorem, Application based on these results. Chemistry Gaseous State : Kinetic theory, molecular velocity, Probable distribution of velocities, mean free path, collision frequency. Distribution of energies of molecules translational, rotational & vibrational, Law of equipartitions of energies, Equation of State of a real gas. Critical phenomenon & principle of corresponding states. The phase rule : Derivation of phase rule, significance of various terms involved in the definition of phase rule. Phase diagrams of one component systems ( Water, Sulphur and CO2 ). Two component system : Eutectic, congruent and incongruent systems with examples : Partial miscible liquids: Lower and upper consolute point. Chemical thermodynamics : Intensive and extensive variables; state and path functions; isolated, closed and open systems; zeroth law of thermodynamics. First law : Concept of heat, q, work, w, internal energy U and statement of first law; enthalpy, H, relation between heat capacities, calculations of q, w, U and H for reversible, irreversible and free expansion of gases ( ideal and van der Waals ) under isothermal and adiabatic conditions. Thermochemistry : Heats of reactions: standard states; enthalpy of formation of molecules and ions and enthalpy of combustion and its applications; calculation of bond energy, bond dissociation energy and resonance energy from thermochemical data, effect of temperature ( Kirchoff’s equations ) and pressure on enthalpy of reactions. Adiabatic flame temperature, explosion temperature. Second Law : Concept of entropy; thermodynamic scale of temperature, statement of the second law of thermodynamics; molecular and statistical interpretation of entropy. Calculation of entropy change for reversible and irreversible processes. Third Law : Statement of third law, concept of residual entropy, calculation of absolute entropy of molecules. Free Energy Functions : Gibbs and Helmholtz energy; variation of S, G, A with T, V, P; Free energy change and spontaneity. Relation between Joule-Thomson coefficient and other thermodynamic parameters; inversion temperature; Gibbs-Helmholtz equation; Maxwell relations; thermodynamic equation of state. Chemical Kinetics : Rate, mechanism, steady state concept, Kinetics of complex reactions, concept of energy barrier / energy of activation. Theories of reaction rates, Lindemann theory of unimolecular reaction and reactions in flow system. Electrochemistry : Concept of electrolysis, Electrical current in ionic solutions. Kohlrausch’s law and migration of ions. Transference number. Hittroff and moving boundary methods. Applications of conductance measurements. Strong electrolytes : Onsager equation: Activity and activity coefficients of strong electrolyte. Surface Chemistry : Adsorption, adsorbate and adsorbents. Types of adsorption. Freundlich adsorption isotherm, Langmuir adsorption isotherms. B.C.T. Isotherm: Surface area of the adsorbent. Changes in entropy, enthalpy and free energy on adsorption. Gibbs adsorption equation. Catalysis : Types of catalysis, homogenous / heterogeneous, enzyme catalysis, acid / base catalysis and their kinetics. Mechanism of heterogeneous catalysis. Kinetics of surface reactions : unimolecular and bimolecular. pH-dependence of rate constants of catalysed reactions. Autocatalysis. Colloids : Colloidal state, classification of colloidal solution, true solution, colloidal solution and suspensions, preparation of sol, Purification of colloidal solutions. viscosity & plasticity General and optical properites, stability f colloids, coagulation of lyphobic sols, electrical properties of sols, kinetic properties of colloids:- Brownion movement, size of colloidal particle, emulsions, gels, colloidal electrolytes and applications of colloids. Emulsions, emulsifiers, theory of emulsification Polymers : Basic concepts & Terminology, such as monomers, Polymers, Functionality, Thermoplastics, Thermosets Linear, Branched, cross linked polymers etc. different definitions of molecular weight viz., Mw, Mn, Mv and then determinations. Industrial applications of polymers, Addition, condensation and Ionic polymerization’s, solutions of polymers, good solvents, & bad solvent, solubility parameter, solutions viscosity and determination of intrinsic viscosity. Atomic Structure : Introduction to wave mechanics, the Schrodinger equation as applied to hydrogen atom, origin of quantum numbers, Long form of periodic table on the basis of Electronic configuration s, p, d, f block elements periodic trends, Ionisation potential, atomic and ionic radii electron affinity & electro-negativity. Chemical Bonding : Ionic bond-energy changes, lattice energy Born Haber Cycle, Covalent bond-energy changes, Potential energy curve for H2 Molecule, characteristics of covalent compound. Co-ordinate bond – Werner’s Theory, effective atomic numbers, isomerism in coordinate compounds. Hydrogen bonding. Concept of hybridisation and resonance, Valance Shell Electron Repulsion theory ( VSEPR ). Discussion of structures of H2O, NH3, SiF4. Molecular orbital theory, Linear combination of atomic orbitals ( LCAO ) method. Structure of simple homo nuclear diatomic molecule like H2, N2, O2, F2. Acids & Bases : Basics of acidities and basicities, electrolytic dissociation, concept of strengths of acids and bases, ionization of water, concept of pH and its scale, Buffer solutions, Buffer solution of weak acid and its salt, calculation of pH of buffer solution, Henderson equation, acid-base indicators and theory of indicators. Classification of Organic compounds IUPAC nomenclature, Structural isomerism, Cis-trans isomerism, shapes and molecular orbital structures of compounds containing C, N and O conformation of alkanes, structures of dienes, pyridine, pyrrole, aromatic compounds, delocalisation, concept of aromaticity, stability of cycloalkanes, resonance concept, inductive and mesomeric effects, directive effects, activating and deactivating groups, hydrogen-bonding, organic reagents and reaction intermediates. Chemistry of hydrocarbons House synthesis halogenation of alkanes, free radical mechanism, cracking effect of structure on Physical properties of compounds, alkenes catalytic hydrogenation, dehydration of alcohols, dehydrogenation, Saytzeff rule, electrophilic addition reactions, peroxide effect, mechanism of allylic substitution, acidity of 1-alkynes, conjugated dienes, 1, 2 and 1, 4 additions, free radical and ionic mechanisms of addition polymerisation reactions. Ring opening reactions of cyclopropane and cyclobutane, chemistry of benzene and alkyl benzenes. Aromatic electrophillic substitution reaction, Friedel-Crafts reaction. Chemistry of functional groups Alkyl and aryl halides, nucleophilic substitution, synthetic utility of Grignard reagents and alkallithiums, Mechanism of Gringnartion of alcohols, Benzyl alcohol, acidity of phenols, Epoxy compounds, Anisole nucleophilic addition, Benzaldehyde, acetophene, benzophenone, aldol condensation, acidity of acids, alkyl and aryl amines. Biology Origin of Life : History of earth, theories of origin of life nature of the earliest organism. Varieties of life : Classification, Five kingdoms, viruses ( TMV, HIV, Bacteriophage ), Prokaryote ( Bacteria-cell structure, nutrition, reproduction ), Protista, Fungi, Plantae and Animalia. Chemicals of life : ( Biomolecules ) – Carbohydrates lipids, amino acids, proteins, nucleic acids, and identification of biomolecules in tissues. Cell : The cell concept, structure of prokaryotic and eukaryotic cells, plant cells and animal cells, cell membrances, cell organelles and their function. Structure and use of compound microscope. Histology : Maritimes ( apical, intercalary, lateral ) and their function; simple tissue ( parenchyma, collenchymas, sclerenchyma ); Complex tissue ( xylem and phloem ); Tissue systems ( epidermal, ground, vascular ); primary body and growth ( root, stem, leaf ); Secondary growth. Animal Epithelial tissue, connective tissue, muscle tissue and nervous tissue and their function in body. Nutrition : Autotrophic ( Photosynthesis ) Pigment systems, Chloroplast, light absorption by chlorophyll and transfer of energy, two pigment systems, photosynthetic unit, phosphorylation and electron transport system, Calvin-Benson Cycle (C3), Hatch Slack Pathway (C4), Crassulacan Acid Metabolism (CAM), factors affecting photosynthesis; Mineral Nutrition in plants. Heterotrophic – Forms of heterotrophic nutrition, elementary canal in humans, nervous and hormonal control of digestive systems, fate of absorbed food materials; Nutrition in humans, Reference values. Energy Utilization : ( Respiration ) - Structure of mitochondria, cellular respiration, relationship of carbohydrate metabolism to other compounds, Glycolysis, fermentation, formation of acetyl co-A, Kreb cycle, Electron Transport System and Oxidative Phosphorylation, ATP, factors affecting respiration. Transport : Plant water relationships, properties of water, diffusion, osmosis, imbibition, movement of water in flowering plants, uptake of water by roots, the ascent of water in xylem, apoplast symplast theory, Transpiration-structure of leaf and stomata in plants opening and closing mechanism of stomata factors affecting transpiration significance of transpiration General characteristics of blood vascular system, development of blood systems in animals, Composition of blood, circulation in blood vessels, formation of tissue fluids, the heart, functions of mammalian blood, the immune system. Topics Origin of Life History of earth, theories of origin of life, nature of earliest organism. Diversity of Life Basic rules of classification and nomenclature, Classification-two kingdom, five kingdom - brief introduction to kingdoms, three domain introduction and structure of viriods, prions and virus ( HIV, TMV, Bacteriophage ), Structure and reproduction of bacteria and their economic importance Chemical basis of life Biomolecules-carbohydrates, proteins, fats and lipids, nucleic acids ( DNA and RNA ) Enzymes Definition, Properties, Types, Mechanism of action, factors affecting kinetics and their industrial applications Cell-Structure and function Prokaryotic and eukaryotic cells, plant and animal cells, structure and function of cell membrane, nucleus, chloroplast, mitochondria, golgi apparatus, endoplasmic reticulum Histology Plant Meristem ( apical, intercalary and lateral ), simple tissue ( parenchyma, collenchymas, and sclerenchyma ), complex tissue ( xylem and phloem ) - structure and function; tissue systems ( epidermal, ground and vascular ); primary body and growth ( root, stem and leaf ), secondary growth. Animals Epithelial, connective, muscular and nervous tissue - structure and function Economic Biology Food - Cereals ( wheat, rice, maize ), Beverages ( tea, coffee, cocoa ), sugarcane, medicinal plants ( Taxus, Catharanthus, Salix, Azadirachta ); and rubber ( Hevea ) Apiculture, Sericulture, Vermiculture and Leather Syllabus for CET for M.Tech ( Engineering Physics ) Programme Physics Interference : Young’s double slit experiment, Fresnel’s biprism, Thin films, Newton’s rings, Michelson’s interferometer, Fabry Perot interferometer. Diffraction : Fresnel Diffraction: Zone plate, circular aperture, opaque circular disc, narrow slit, Fraunhofer diffraction : Single slit, double slit, diffraction grating, resolving power and dispersive power. Polarization : Types of polarization, Brewsters law, Malu’s Law, Nicol prism, double refraction, quarter-wave and half-wave plates, optical activity, specific rotation. Lasers : Introduction, coherence, population inversion, basic principle and operation of a laser, Einstein A and B coefficients, type of lasers, He-Ne laser, Ruby laser, semiconductor laser, holography-theory and applications Fibre Optics : Types of optical fibres and their characteristics, ( Attenuation and dispersion step index and graded index fibres, principle of fibre optic communication-total internal reflection, numerical aperture, fibre optical communication network ( qualitative )-its advantages. Theory of Relativity : Galenlian transformations, the postulates of the special theory of relativity, Lorentz transformations, time dilation, length contraction, velocity addition, mass energy equivalence. Thermodynamics : The first law and other basic concepts: dimensions, units, work, heat, energy, the first law of thermodynamics, enthalpy, equilibrium, phase rule, heat capacity, PVT behavior of pure substances, ideal gas, real gas, heat effects. The second law and Entropy : statements, heat engines, Kelvin-Planck and Clausious statements and their equality, reversible and irreversible processes, Carnot cycle, thermodynamic temperature scale, entropy, ent ropy calculations, T-S diagrams, properties of pure substances, use of steam tables and Mollier diagram. Refrigeration and liquefaction : the Carnot refrigerator, the vapor–compression cycle, comparison of refrigeration cycles, liquefaction processes, heat pump. Rankine power cycle. Quantum Mechanics : Wave particle duality, deBroglie waves, evidences for the wave nature of matter – the experiment of Davisson and Germer, electron diffraction, physical interpretation of the wave function and its properties, the wave packet, the uncertainty principle. The Schrodinger wave equation ( 1 – dimensional ), Eigen values and Eigen functions, expectation values, simple Eigen value problems – solutions of the Schrodinger’s equations for the free particle, the infinite well, the finite well, tunneling effect, simple harmonic oscillator ( qualitative ), zero point energy. Quantum Statistics : The statistical distributions, Maxwell Boltzmann, Bose-Einstein and Fermi-Dirac statistics, their comparisons, Fermions and Bosons. Applications: Molecular speed and energies in an ideal gas. The Black-body spectrum and failure of classical statistics to give the correct explanation – the application of Bose-Einstein statistics to the Black-body radiation spectrum, Fermi-Dirac distribution to free electron theory, electron specific heats, Fermi energy and average energy – its significance. Band theory of solids : Origin of energy bands in solids, Kronig-Penny model, Brillouin zones, effective mass, Metals, semiconductors and insulators and their energy band structure. Extrinsic and intrinsic semiconductors, p-n junction diodes- its characteristics, tunnel diode, zener diode, photodiode, LED, photovoltaic cell, Hall effect in semiconductors, transistor characteristics ( common base, common emitter, common collector ). Digital techniques and their applications ( registers, counters, comparators and similar circuits ) A/D and D/A converters. Superconductivity : ZFC and FC, Meissner effect, Type I and II superconductors, the Josephson effect, flux quantization, Cooper pairs, BCS theory, properties and applications of superconductors. X-rays : production and properties, crystalline and amorphous solids, Bragg’s law, applications. Electricity and magnetism : Electric fields, Gauss’ Law, its integral and differential form, applications. Lorentz force, fields due to moving charges, the magnetic field, Ampere’s law, motion of a charged particle in an electric and magnetic field, magnetic and electrostatic focussing, Hall effect, determination of e/m by cathode ray tube, positive rays, Thomson’s parabolic method, Isotopes, Mass spectrographs ( Aston and Bainbridge ), Electron microscope, Cyclotron and Betatron. Overview of Electro – Magnetism : Maxwell’s Equations: The equation of continuity for Time – Varying fields, Inconsistency in ampere’s law Maxwell’s Equations, conditions at a Boundary Surface, Introduction to EM wave. Nuclear Physics : Introduction of nucleus, Nucleus radius and density, Nuclear forces, Nuclear reactions, Cross section, Q-value and threshold energy of nuclear reactions, Basic Idea for Nuclear Reactor, Breeder reactor, The Geiger-Mullar ( G.M. ) Counter, Introduction of Accelerators and its Applications. Numerical Techniques : Interpolations, differentiation, integration; Nonlinear equations, the bisection methods, Newton’s method, root finding; Differential equations, Euler’s method, the Runge-Kutta method; Matrices-inverting, finding eigenvalues and eigenfunctions. Mathematics Linear Independence and dependence of vectors, Systems of linear equations – consistency and inconsistency, rank of a matrix, Gauss elimination method, , Eigen values and Eigen vectors. Successive differentiation, Leibnitz’s theorem, Lagrange’s Theorem, Cauchy Mean value theorems, Taylor’s theorem, Asymptotes, Curvature, Reduction Formulae of trigonometric functions, Properties of definite Integral, Applications to length, area, volume, surface of revolution. Partial derivatives, Method of Lagrange’s multipliers. Jacobeans of coordinates transformations. Double and Triple integrals. Method of separation of variables, homogeneous, linear equations, exactness and integrating factors, linear equations of higher order with constant coefficients, Operator method to find particular integral. Scalar and vector fields, Directional Derivative, Gradient of scalar field, divergence and curl of a vector field. Green’s theorem, Divergence theorem and Stoke’s theorem. Probability : Definition of Sample Space, Event, Event Space, Conditional Probability, Additive and Multiplicative law of Probability, Baye’s Law theorem, Application based on these results. |