|
#2
24th July 2014, 10:54 AM
| |||
| |||
| Re: Previous NET Chemistry Exam paper
Here I am giving you question paper for ICAR NET chemistry examination in a PDF fiel attached with it . so you can get it easily 1. (a) What is Zeise's salt? Show its structure and describe the bonding. (5) (b) Use Coulomb's law to deduce the dimension of charge. (5) (c) Explain which of the following is primarily responsible for the difference in chemical properties of nanomaterials from the bulk. (2) i. lattice defects ii. surface to volume ratio iii. London forces (d) Suggest a synthetic method (may need more than one step) for the following transformation. (5) MeO MeO (e) Match the following: (3) Compounds Type 1. progesterone i. antiulcer compound 2. amoxycillin ii. antiallergic 3. morphine iii. antibacterial iv. hallucinogen v. harmone 2. (a) Identify the products A to E in the following reactions. (9) (i) W(CO)6 + LiPh → A Me3O+BF4 - B Cl Cl C + Fe2(CO)9 (ii) (iii) [Ph3C3][X] + Ni(CO)4 → D (iv) (η5-Cp)2Fe + CH3COCl → E (b) Draw the structures of Co4(CO)12 and Ir4(CO)12. (6) 3. (a) Write appropriate equations for the synthesis of (i) N3P3Cl6 and (ii) N3P3F6. (c) Sketch all isomers of PF3(CH3)2 assuming a trigonal bipyramidal geometry in each case. (6) 4. (a) What is the origin of the blue colour in Prussian Blue? Comment on the effect of (i) oxidant and (ii) reductant on its colour. (7) (b) Give an example of a diamagnetic transition metal hydride complex. How is the presence of metal bound hydride detected by spectroscopic methods. (6) (c) A Rh(PPh3)3Cl THF, H2 Draw the structure of A above, showing the stereochemistry. (2) 5. (a) The complex ion [Co(tren)NH3Cl]2+ (tren = N(CH2CH2NH2)3) exists as red and purple isomers. Draw the structures of these two forms. (4) (b) Red isomer undergoes hydrolysis much more rapidly compared to the purple one. Explain 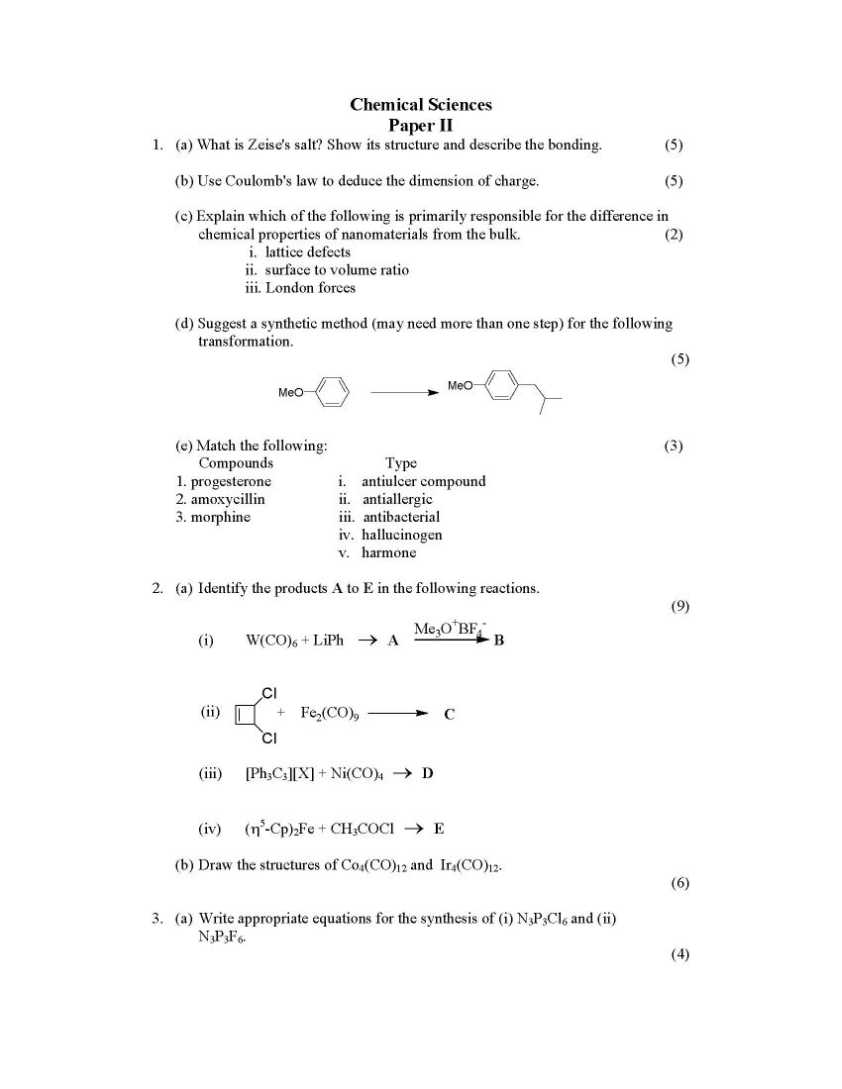      |