|
#2
22nd December 2014, 12:45 PM
| |||
| |||
| Re: ISAT Exam study material
As you said you have have filled form for appear in ISAT admission exam for doing B.tech , so as on your demand I am providing both syllabus and question paper : Syllabus : ISAT admission exam syllabus : PHYSICS MECHANICS Units and Measurements: The international system of units, measurement of length, mass and time, accuracy, precision of instruments and errors in measurement, Significant figures, dimension of physical quantities, dimensional formulae and equations, dimensional analysis and its applications. Motion in a straight line: position, path length and displacement, average velocity and speed, instantaneous velocity and speed, acceleration, kinematic equations for uniformly accelerated motion, relative velocity. Motion in a plane: scalars and vectors, multiplication of vectors by real numbers, addition and aubtraction of vectors- graphical method, resolution of vectors, vector addition – analytical method, motion in a plane, motion in a plane with constant acceleration, relative velocity in two dimensions, projectile motion, uniform circular motion. Laws of motion: the law of inertia, Newton’s first, second and third law of motion, conservation of momentum, equilibrium of particle, common forces in mechanics, circular motion. Work, Power and Energy: the work energy theorem, kinetic and potential energy, work-energy theorem for variable force, the conservation of mechanical energy, Power, the potential energy of a spring, collisions. System of particles and rotational motion: centre of mass, motion of centre of mass, linear momentum of a system of particles, vector product of two vectors, angular velocity and linear velocity relations, torque and angular momentum, equilibrium of a rigid body, Moment of Inertia, theorem of perpendicular and parallel axes, kinematics and dynamics of rotational motion about a fixed axis, angular momentum in case of rotation about a fixed axis, rolling motion Gravitation: Kepler’s laws, universal law of gravitation, gravitation constant, acceleration due to gravity of the earth, acceleration due to gravity below and above the surface of earth, gravitational potential energy. ELETROMAGNETISM Electric charges and Fields: Electric charges, conductors and insulators, basic properties of electric charge, Coulomb’s law, Force between multiple charges, electric field and flux, electric dipole, continuous charge distribution, Gauss’s law and its applications. Electrostatic Potential and capacitance: electrostatic potential, potential due to a point charge and systems of charges, potential due to an electric dipole, equipotential surfaces, Potential energy in an external field, electrostatics of conductors, dielectric and polarization, capacitors and capacitance, the parallel plate capacitor, combination of capacitors, energy stored in a capacitor. Current Electricity: electric current, electric currents in conductors, Ohm’s law, drift of electrons and origin of resistivity, resistivity and its temperature dependence, electrical energy and power, Combination of resistors (Series and Parallel) , cells, emf, internal resistance, cells in series and parallel, Kirchoff’s laws, Wheatstone bridge, meter bridge, and potentiometer. Moving charges and Magnetism: magnetic force, motion in a magnetic field, motion in a combined electric and magnetic fields, magnetic field due to a current element and Biot-Savart law, magnetic field on the axis of a circular current loop, Ampere’s circuital law, the solenoid and toroid, force between, two parallel currents, torque and current loop and magnetic dipole, the moving coil galvanometer. Magnetism and Matter: the bar magnet, the earth’s magnetism, magnetic properties of materials, permanent magnets and electromagnets. Electromagnetic induction: magnetic flux, Faraday’s law of induction, Lenz’s law and conservation of energy, motional electromagnetic force, energy consideration: a quantitative study, inductance and AC generator. Alternating current: AC Voltage applied to a resistor, an inductor, a capacitor, Ac Voltage applied to a series LCR circuit, power in AC Circuit, LC oscillations, transformers. OPTICS AND WAVES Ray Optics and Optical Instruments: reflection of light by spherical mirrors, refraction, total internal reflection, refraction at spherical surfaces and by lenses, refraction through a prism, dispersion by a prism, some natural phenomenon due to a sunlight, optical instruments, Wave Optics: Huygens Principle, Refraction and reflection of plane waves using Huygens principle, coherent and incoherent addition of waves, interference of light waves and Young’s experiment, diffraction, polarisation Oscillations: periodic and oscillatory motions, simple harmonic motion and uniform circular motion, velocity and acceleration in simple harmonic motion, force and energy in simple harmonic motion, damped SHM and forced oscillations and resonance Waves: transverse and longitudinal waves, displacement and speed of a travelling wave, principle of superposition of waves, reflection of waves, beats, Doppler Effect Dual nature of radiation and matter: photoelectric effect, wave theory of light and particle nature of light, wave nature of matter PROPERTIES OF MATTER, THERMODYNAMICS Mechanical properties of solids: elastic behaviour of solids, stress and strain, Hooke’s law, applications of elastic behaviour of materials Mechanical properties of fluids: pressure, streamline flow, Bernoulli’s principle, viscosity, Reynold’s number, surface tension Thermal properties of matter : temperature and heat, measurement of temperature, Ideal-gas equation and absolute temperature, thermal expansion, specific heat capacity, calorimetry, change of state, heat transfer, Newton’s law of cooling Thermodynamics: thermal equilibrium, Zeroth law of thermodynamics, heat, internal energy and work, first law of thermodynamics, specific heat capacity, thermodynamic state variables and equation of state, thermodynamic processes, heat engines, refrigerators and heat pumps, second law of thermodynamics, reversible and irreversible processes, carnot engine Kinetic theory: molecular nature of matter, behavior of gases, kinetic theory of an ideal gas, law of equipartition of energy, mean free path Laboratory related questions vernier calipers, screw gauge measurements, traveling microscopes, spectrometers, meter bridges, potentiometers and Wheatstone bridge, minimum deviation measurements, refraction and reflection of light experiments etc, galvanometer, ammeter, voltmeter CHEMISTRY INORGANIC CHEMISTRY Basic Concepts of Chemistry: particulate nature of matter, laws of chemical combination, Dalton’s atomic theory: concept of elements, atoms and molecules. atomic and molecular masses, molecular formula, stoichiometry. Structure of Atom: atomic number, isotopes and isobars. different atomic models and limitations, shells and sub-shells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, orbitals, quantum numbers, shapes of s, p, and d orbitals, Aufbau principle, Pauli exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half filled and completely filled orbitals. Classification of Elements and Periodicity in Properties : periodic table, periodic trends in properties of elements Chemical Bonding and Molecular Structure: valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, hybridization involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules Hydrogen: Occurrence, isotopes, preparation, properties and uses of hydrogen and its compounds. s-Block Elements (Group 1 and Group 2 elements): electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties and in chemical reactivity, uses. preparation and properties of compounds of Na, Ca, Mg and their biological importance. p-Block Elements : general Introduction to p-Block Elements Elements of Group 13, 14 15,16, 17and 18: electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous properties of first element of the group. chemical and physical properties of boron, aluminium, carbon, silicon, nitrogen, phosphorous, oxygen, sulphur, halogens and important compounds of the elements. d and f Block Elements : electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals. General Principles and Processes of Isolation of Elements : concentration, oxidation, reduction electrolytic method and refining; occurrence and principles of extraction of aluminium, copper, zinc and iron. Lanthanides: electronic configuration, oxidation states, chemical reactivity and lanthanide contraction. Actinides: electronic configuration, oxidation states. Coordination compounds: Ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds, bonding; isomerism, importance of coordination compounds. PHYSICAL CHEMISTRY States of Matter : three states of matter, intermolecular interactions, type of bonding, melting and boiling points, molecular, ionic, covalent and metallic solids, amorphous and crystalline solids, unit cell in two dimensional and three dimensional lattices, calculation of density of unit cell, packing in solids, voids, number of atoms per unit cell in a cubic unit cell, point defects, electrical and magnetic properties. Boyle’s law, Charles’ law, Gay Lussac’s law, Avogadro’s law, ideal behaviour, empirical derivation of gas equation, Avogadro’s number, ideal gas equation, deviation from ideal behaviour, liquefaction of gases, critical temperature. liquid State. Solutions : types of solutions, solubility of gases in liquids, solid solutions, colligative properties – relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses Thermodynamics : systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. first law of thermodynamics – internal energy and enthalpy, heat capacity and specific heat, measurement of ΔU and ΔH, Hess’s law of constant heat summation, enthalpy of: bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, and dilution. entropy as a state function, free energy change for spontaneous and nonspontaneous process, equilibrium. Equilibrium : equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle; ionic equilibrium –ionization of acids and bases, strong and weak electrolytes, degree of ionization, concept of pH. Hydrolysis of salts, buffer solutions, solubility product, common ion effect. Redox Reactions : redox reactions, oxidation number, balancing redox reactions, applications of redox reactions. Electrochemistry : conductance in electrolytic solutions, specific and molar conductivity variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and laws of electrolysis, dry cell – electrolytic cells and Galvanic cells; lead accumulator, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, fuel cells; corrosion. Chemical Kinetics : rate of a reaction, factors affecting rates of reaction, order and molecularity of a reaction; rate law and specific rate constant, integrated rate equations and half life (only for zero and first order reactions); concept of collision theory. Surface Chemistry : physisorption and chemisorption; factors affecting adsorption of gases on solids; catalysis: homogenous and heterogeneous, activity and selectivity: enzyme catalysis; colloidal state: distinction between true solutions, colloids and suspensions, Tyndall effect, Brownian movement, electrophoresis, coagulation; emulsions – types of emulsions. Nuclear chemistry: radioactivity: isotopes and isobars; Properties of α, β, and γ rays; Kinetics of radioactive decay (decay series excluded), carbon dating; Stability of nuclei with respect to proton-neutron ratio; fission and fusion reactions. ORGANIC CHEMISTRY Basic Principles and Techniques : methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions; electrophiles and nucleophiles, types of organic reactions. Hydrocarbons: Alkanes, alkenes and alkynes: nomenclature, isomerism, physical properties, methods of preparation. Conformations (ethane only), structure of double bond (ethene), geometrical isomerism, structure of triple bond (ethyne), chemical reactions. Aromatic hydrocarbons: Introduction, IUPAC nomenclature; Benzene: resonance, aromaticity; chemical properties: mechanism of electrophilic substitution, influence of functional group in mono-substituted benzene. Haloalkanes and haloarenes: nomenclature, nature of C-X bond, physical and chemical properties, mechanism of substitution reactions, environmental effects of compounds Alcohols, Phenols and Ethers : Nomenclature, methods of preparation, physical and chemical properties, uses. Identification of primary, secondary and tertiary alcohols, mechanism of dehydration. Acidic nature of phenol, electrophilic substitution reactions. Aldehydes, Ketones and Carboxylic Acids Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties. Mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, acidic nature of carboxylic acids. Organic Compounds Containing Nitrogen: Amines, cyanides, isocyanaides and diazonium salts OTHER TOPICS OF IMPORTANCE Environmental Chemistry : Environmental pollution : Air, water and soil pollution, green chemistry, control of environmental pollution. Biomolecules ; carbohydrates, proteins, vitamins, Nucleic Acids Polymers : natural and synthetic polymers, methods of polymerization, copolymerization. Polymers like polythene, nylon, polyesters, bakelite, rubber. Chemistry in Everyday Life ; Chemicals in medicines, chemicals in food, cleansing agents and action. MATHEMATICS PERMUTATIONS AND COMBINATIONS Fundamental principle of counting. Permutations and Combinations, derivation of formulae and their connections and simple applications. MATHEMATICAL INDUCTION Principle of Mathematical Induction and its simple applications. BINOMIAL THEOREM AND ITS SIMPLE APPLICATIONS Binomial theorem for positive integral indices, general term and middle term, properties of Binomial coefficients and simple applications. SEQUENCES AND SERIES Arithmetic and Geometric progressions, insertion of arithmetic, geometric means between two given numbers. Relation between A.M. and G.M. Sum upto n terms of special series n, n2, n3. Arithmetico - Geometric sequence. TRIGONOMETRY Trigonometric functions. Trigonometrical identities and equations. Inverse Trigonometric functions, their properties and applications. COMPLEX NUMBERS AND QUADRATIC EQUATIONS Complex numbers as ordered pairs of reals. Representation of complex numbers in a plane. Argand plane and polar representation of complex numbers. Algebra of complex numbers, modulus and argument (or amplitude) of a complex number, square root of a complex number, triangle inequality. Quadratic equations in real and complex number system and their solutions. Relation between roots and coefficients, nature of roots, formation of quadratic equations with given roots. SETS, RELATIONS AND FUNCTIONS Sets and their representations. Union, intersection and complement of sets and their algebraic properties. Power Set. Relation, types of relations and equivalence relation. One-one, into and onto functions and composition of functions. Real - valued functions, algebra of functions, polynomials, rational, trigonometric, logarithmic and exponential functions, inverse functions. Graphs of simple functions. Even and odd functions. LIMIT, CONTINUITY AND DIFFERENTIABILITY Limit and continuity of a function, limit and continuity of the sum, difference, product and quotient of two functions, L'Hospital rule of evaluation of limits of functions. Differentiability of functions. Differentiation of the sum, difference, product and quotient of two functions. Differentiation of trigonometric, inverse trigonometric, logarithmic, exponential, composite and implicit functions; derivatives of order up to two. Rolle’s and Lagrange’s Mean Value Theorems. Applications of derivatives: rate of change of quantities, monotonic - increasing and decreasing functions, maxima and minima of functions of one variable, tangents and normals. INTEGRAL CALCULUS Integral as an anti-derivative. Fundamental integrals involving algebraic, trigonometric, exponential and logarithmic functions. Integration by substitution, by parts and by partial fractions. Integration using trigonometric identities. Definite Integral as limit of a sum. Fundamental Theorem of Calculus. Properties of definite integrals. Evaluation of definite integrals. Applications of the integrals: determining areas of the regions bounded by simple curves in standard form. DIFFERENTIAL EQUATIONS Ordinary differential equations, their order and degree. Formation of differential equation whose general solution is given. Solution of differential equations by the method of separation of variables. Solution of homogeneous differential equations and linear first order differential equations. CO-ORDINATE GEOMETRY Cartesian coordinate system, distance formula, section formula, locus and its equation, translation of axes, slope of a line, parallel and perpendicular lines, intercepts of a line on the coordinate axes. Straight lines : Various forms of equations of a line, intersection of lines, angles between two lines, conditions for concurrence of three lines, distance of a point from a line, equations of internal and external bisectors of angles between two lines, coordinates of centroid, orthocentre and circumcentre of a triangle, equation of family of lines passing through the point of intersection of two lines. Circles, Conic sections : Standard equation of a circle, general form of the equation of a circle, its radius and centre, equation of a circle when the end points of a diameter are given, points of intersection of a line and a circle with the centre at the origin and condition for a line to be tangent to a circle, equation of the tangent. Sections of a cone, standard equations and properties of conic sections (parabola, ellipse and hyperbola), condition for y = mx + c to be a tangent and point (s) of tangency. THREE DIMENSIONAL GEOMETRY Coordinates of a point in space, distance between two points, section formula. Direction ratios and direction cosines of a line joining two points, angle between two intersecting lines. Coplanar and Skew lines, the shortest distance between two lines. Equations of a line and a plane in different forms, intersection of a line and a plane. VECTOR ALGEBRA Scalars and vectors, addition of vectors, components of a vector in two dimensional and three dimensional spaces, scalar and vector products scalar and vector triple product. MATRICES AND DETERMINANTS Matrices, algebra of matrices, types of matrices, elementary row and column operations. Determinant of matrices of order two and three. Properties of determinants, area of triangles using determinants. Adjoint and inverse of a square matrix. Test of consistency and solution of system of linear equations in two or three variables using inverse of a matrix. STATISTICS AND PROBABILITY Measures of Dispersion: Calculation of mean, median, mode of grouped and ungrouped data. Calculation of standard deviation, variance and mean deviation for grouped and ungrouped data. Probability: Probability of an event, addition and multiplication theorems of probability, Baye’s theorem, probability distribution of a random variable, Bernoulli trials and Binomial distribution. ISAT admission exam question paper  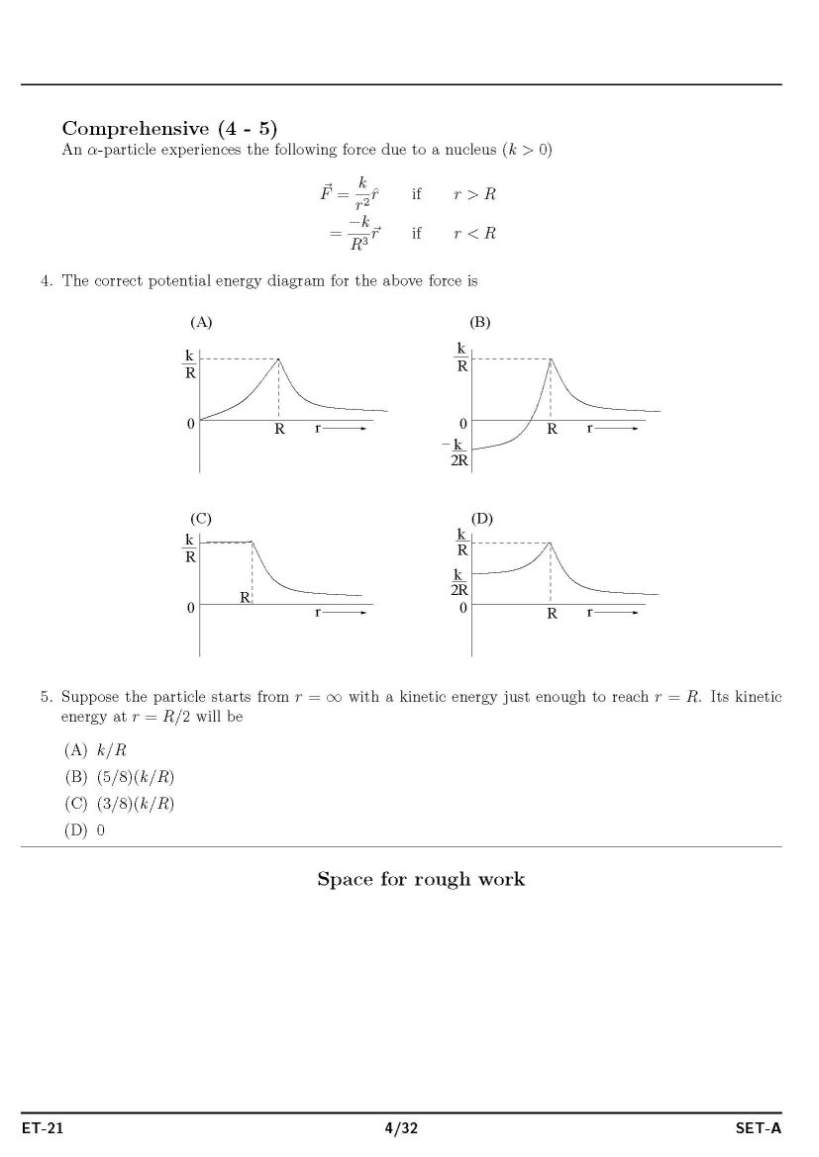 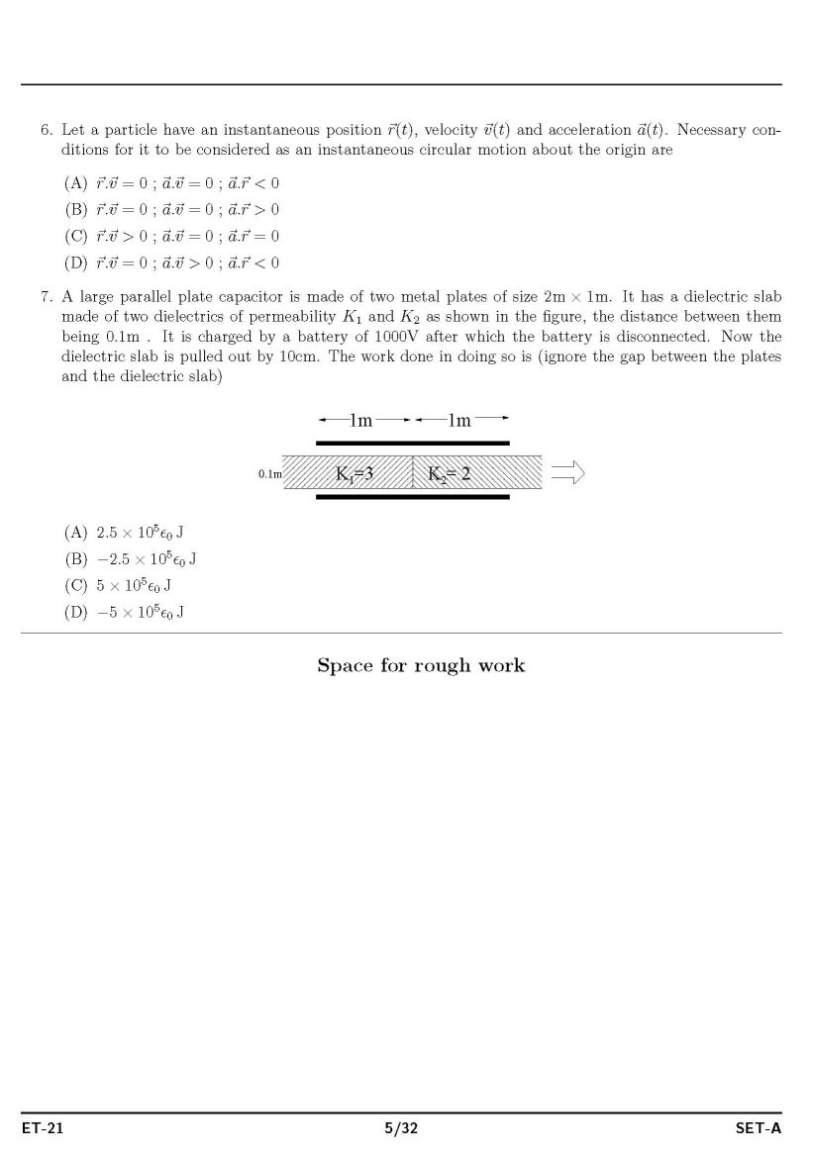 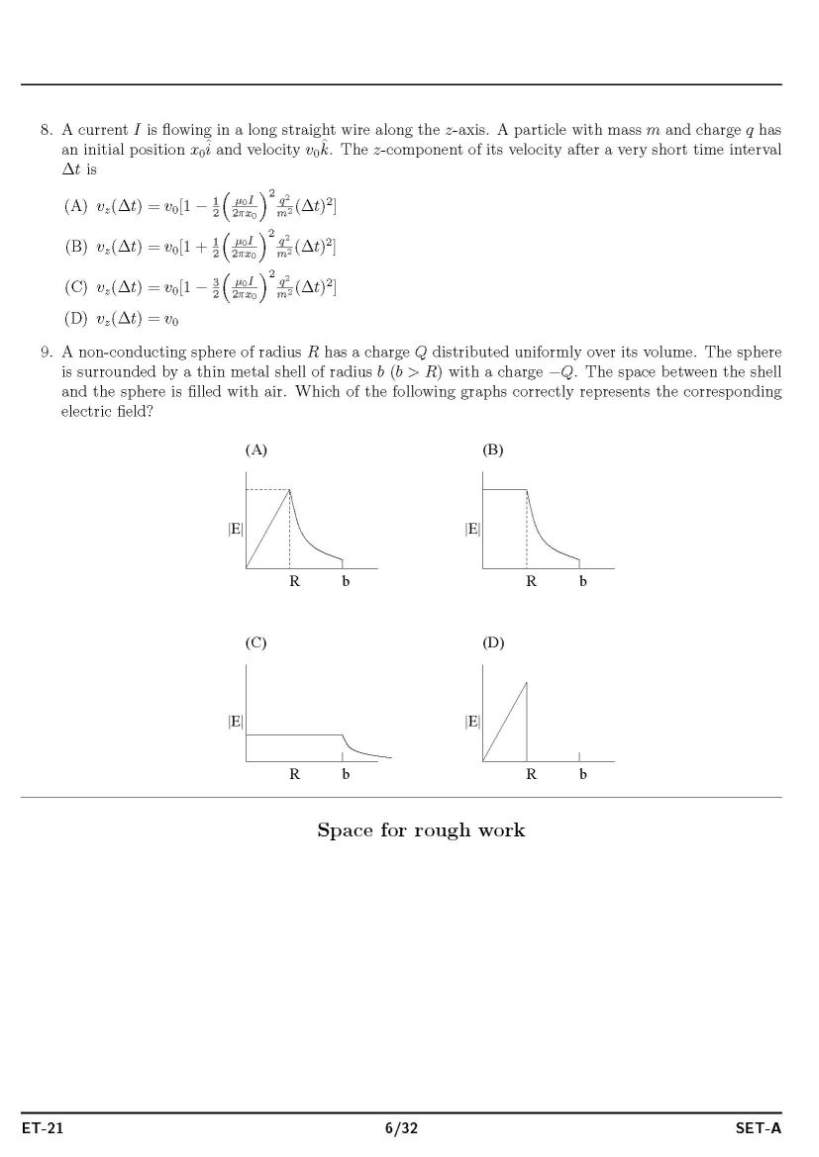 more paper detail to atteched two pdf file.................. |