|
#2
7th August 2014, 01:30 PM
| |||
| |||
| Re: IIT JEE Last 2 Year Papers
Here I am giving you solved question paper for IIT Joint entrance examination in a PDF file attached with it so you can get it easily. 1. The Henry’s law constant for the solubility of N2 gas in water at 298 K is 1.0 × 105 atm. The mole fraction of N2 in air is 0.8. The number of moles of N2 from air dissolved in 10 moles of water at 298 K and 5 atm pressure is (A) 4.0 × 10−4 (B) 4.0 × 10−5 (C) 5.0 × 10−4 (D) 4.0 × 10−6 Sol. (A) 3. The reaction of P4 with X leads selectively to P4O6. The X is (A) Dry O2 (B) A mixture of O2 and N2 (C) Moist O2 (D) O2 in the presence of aqueous NaOH Sol. (B) P4 + 3O2 2 N → P4O6 (exclusively) (N2 is used to retard the further oxidation.) 4. Among cellulose, poly(vinyl chloride), nylon and natural rubber, the polymer in which the intermolecular force of attraction is weakest is (A) Nylon (B) Poly(vinyl chloride) (C) Cellulose (D) Natural Rubber Sol. (D) As chain of natural rubber involves weak van der Waal force of interaction. 5. Given that the abundances of isotopes 54Fe, 56Fe and 57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is (A) 55.85 (B) 55.95 (C) 55.75 (D) 56.05 Sol. (B) 6. The IUPAC name of the following compound is OH CN Br (A) 4-Bromo-3-cyanophenol (B) 2-Bromo-5-hydroxybenzonitrile (C) 2-Cyano-4-hydroxybromobenzene (D) 6-Bromo-3-hdyroxybenzonitrile Sol. (B) Priority of CN is highest. 7. Among the electrolytes Na2SO4, CaCl2, Al2(SO4)3 and NH4Cl, the most effective coagulating agent for Sb2S3 sol is (A) Na2SO4 (B) CaCl2 (C) Al2(SO4)3 (D) NH4Cl Sol. (C) As Sb2S3 is a negative sol, so, Al2(SO4)3 will be the most effective coagulant due to higher charge density on Al3+ in accordance with Hardy-Schulze rule. Order of effectiveness of cations: Al3+ > Ca++ > Na+ > 4 NH+ 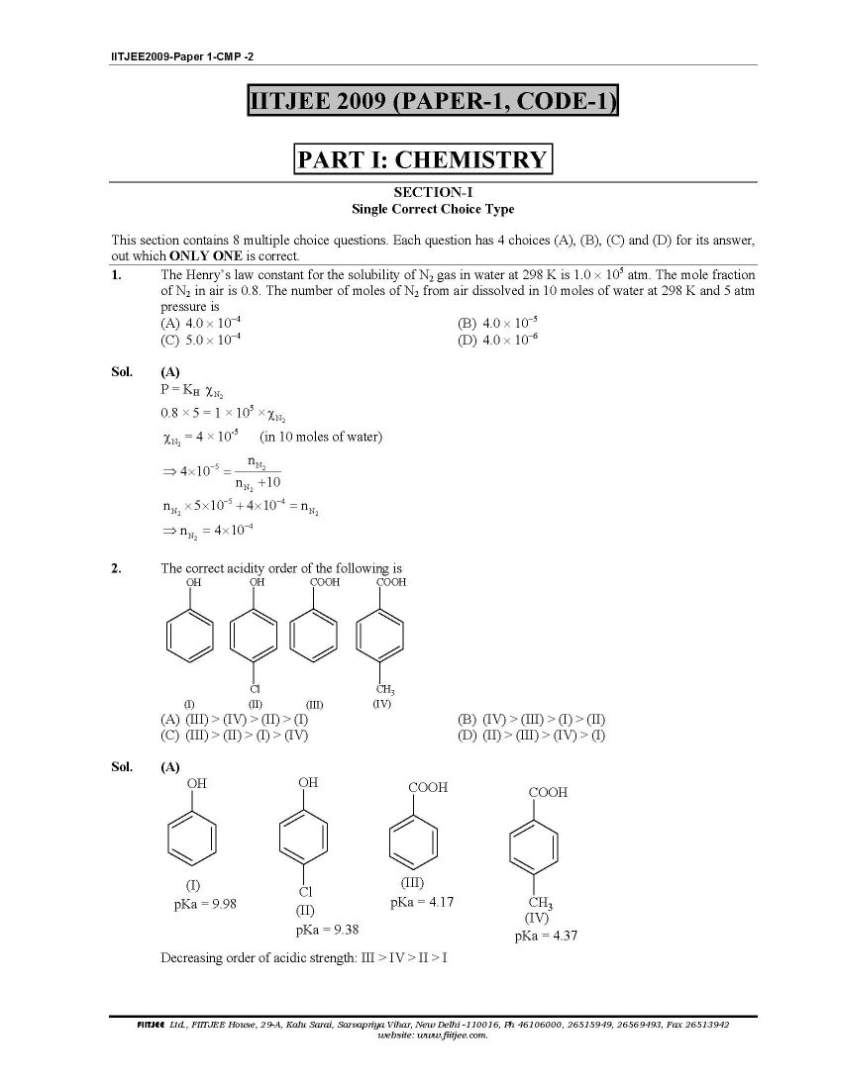 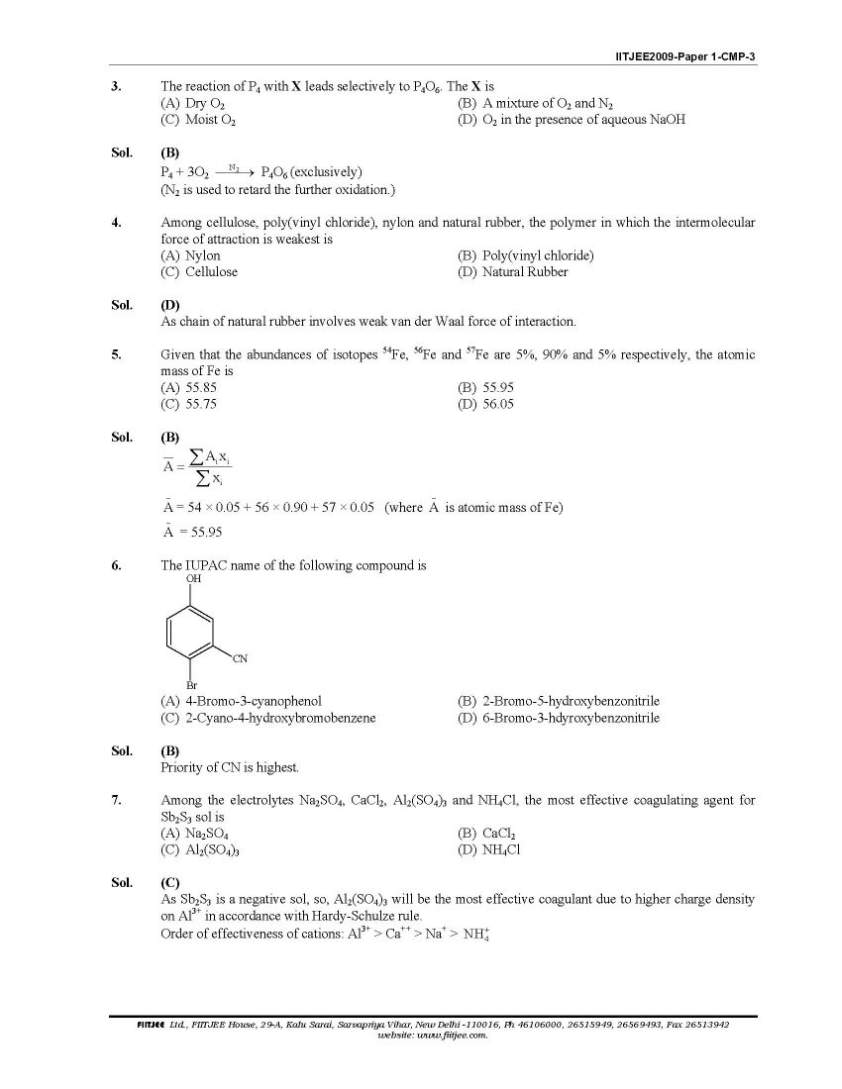 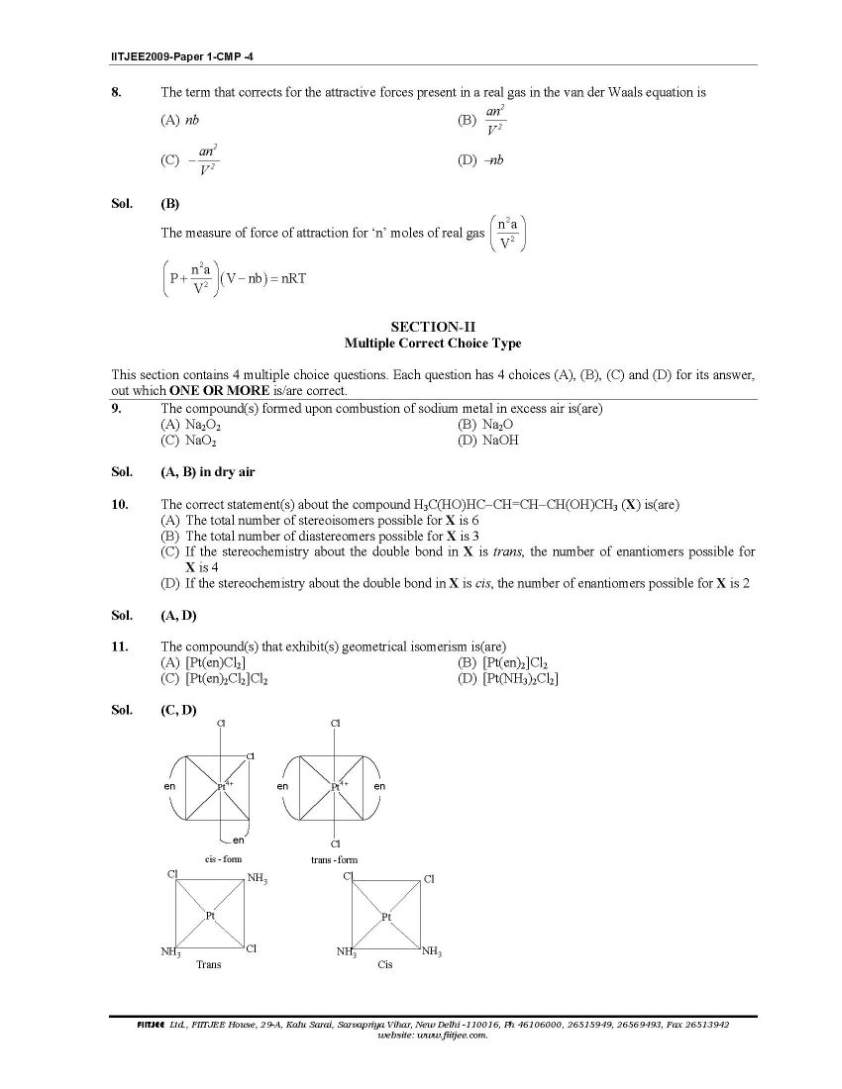 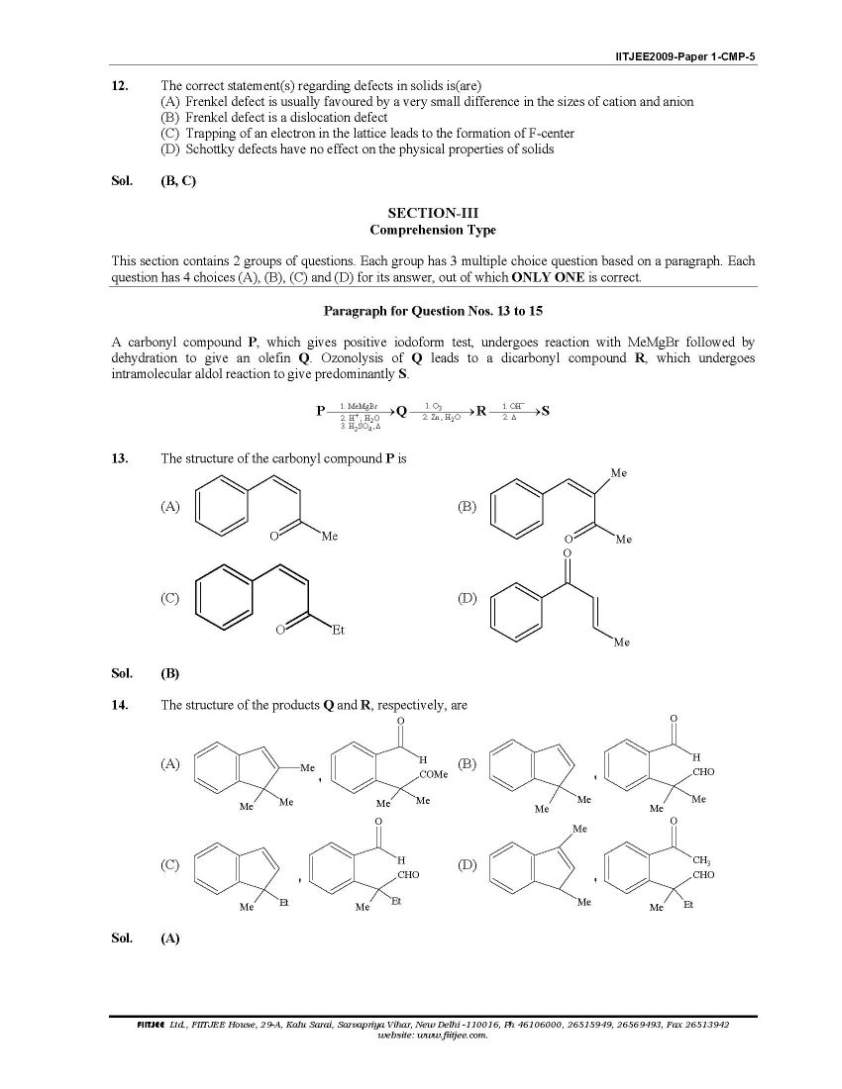  |