|
#3
6th July 2018, 07:48 AM
| |||
| |||
| Re: Gurukul kangri vishwavidyalaya faculty of engineering and technology
The syllabus of B. Tech Computer Science & Engineering Program offered by Faculty of Engineering & Technology of Gurukula Kangri Vishwavidyalaya, Haridwar is as follows: ECH 101 / ECH 201 BAC-C101-ENGINEERING CHEMISTRY UNIT I Periodicity & Chemical Bonding: Atomic radii, Ionization potential, Electro negativity, Electro positivity, Electron affinity and their periodicity. Hybridization involving s, p and d orbital, partial ionic character, dipole moment and its applications, hydrogen bond and Vander Waals forces, elementary treatment of M.O. theory and its application to homo nuclear diatomic molecules of I and II period elements. Phase Rule: Gibbs phase rule (without derivation). Applications of Phase rule to one component system (H2O and S) and two component system (KI- H2O system). UNIT II Chemical kinetics: Arrhenius equation, determination of activation energy, theories of reaction rates (collision and absolute reaction rate theory). Photochemistry: Laws of Photochemistry, Quantum yield, Fluorescence, Phosphorescence, Chemiluminescence, Jabolinski diagram UNIT III Water Analysis: Hard & soft water, Specification of water, Analysis of water-alkalinity, hardness (EDTA Method only) of water for domestic use, Water softening-soda-lime process, anion exchangers, Boiler-feed water, Boiler problems-scale and sludge, priming & forming, Caustic embittlement & corrosion, their cause and prevention (Removal of dissolved gases, carbonate treatment, Phosphate conditioning, Colloidal conditioning), numerical problems based on hardness. Solid impurities (filterable, non-filterable), pH, D.O, B.O.D., C.O.D Polymers: Polymers, thermoplastics, thermosetting plastic, linear, branched & cross linked polymers etc., industrial application of polymers, addition, condensation polymerizations (I)Plastics: Structure, properties and uses of thermoplastic (Polyvinyl chloride, Teflon, Nylons and Polymethyl methacrylate) and thermosetting (Bakelite) materials. (II)Rubber: natural Rubber and its preparations, vulcanization, mechanism of vulcanization, synthetic rubber (General). UNIT IV Fuels: Definition and classification, Calorific value; Gross & Net calorific value and their determination by Bomb calorimeter. (I)Solid fuels: Coke-its manufacture by Otto Hoffman oven and uses. (II) Liquid fuels: Conversion of coal into liquid fuels (Bergius process & Fischer Tropsch process and mechanism), Petroleum- its chemical composition and fractional distillation. Cracking of Heavy oil residues (Thermal cracking and catalytic cracking),Knocking & Anti knocking agents, octane and cetane numbers and their significance. (III)Gaseous fuels: Natural Gas, Producer gas, Water gas, Carburetted water gas, Coal gas and Oil gas. (IV)Nuclear fuels: Nuclear fission and nuclear fusion.Nuclear reactor. Corrosion: Definition and types of corrosion, Electrochemical Theory of corrosion, laws of oxide film, different theories of corrosion, Atmospheric corrosion, stress corrosion water line, pitting and soil corrosion. Protective measures against corrosion 9 UNIT V Lubricants: Principle of Lubrication, types of Lubrication, Lubricating oil, fraction from crude oil, de-waxing of oil fraction, acid and solvent, refining of lubricating oils, properties of refined oils (viscosity, viscosity index, acid value, saponification value & iodine value, pour point and cloud point, flash point and fire point, aniline point, and their determination, Lubricant greases (Semi solid) and their Penetration and drop point tests, solid lubricants. Name Reactions: Reimer Tieman reaction, Aldol Condensation, Diels Alder Reaction, Wurtz Reaction and Claisen Reaction References 1. Principales of Physical chemistry : B.R. Puri, L.R. Sharma, M. Pathania 2. Advanced inorganic chemistry : Cotton 3. A text book of organic chemistry : S.K. Jain 4. Principals of Physical Chemistry : Samuel Glastone 5. A text book of Engineering chemistry : S.S. Dara 6. A text book of Engineering chemistry : Jain Syllabus B.Tech Computer Science & Engineering Gurukula Kangri Vishwavidyalaya, Haridwar 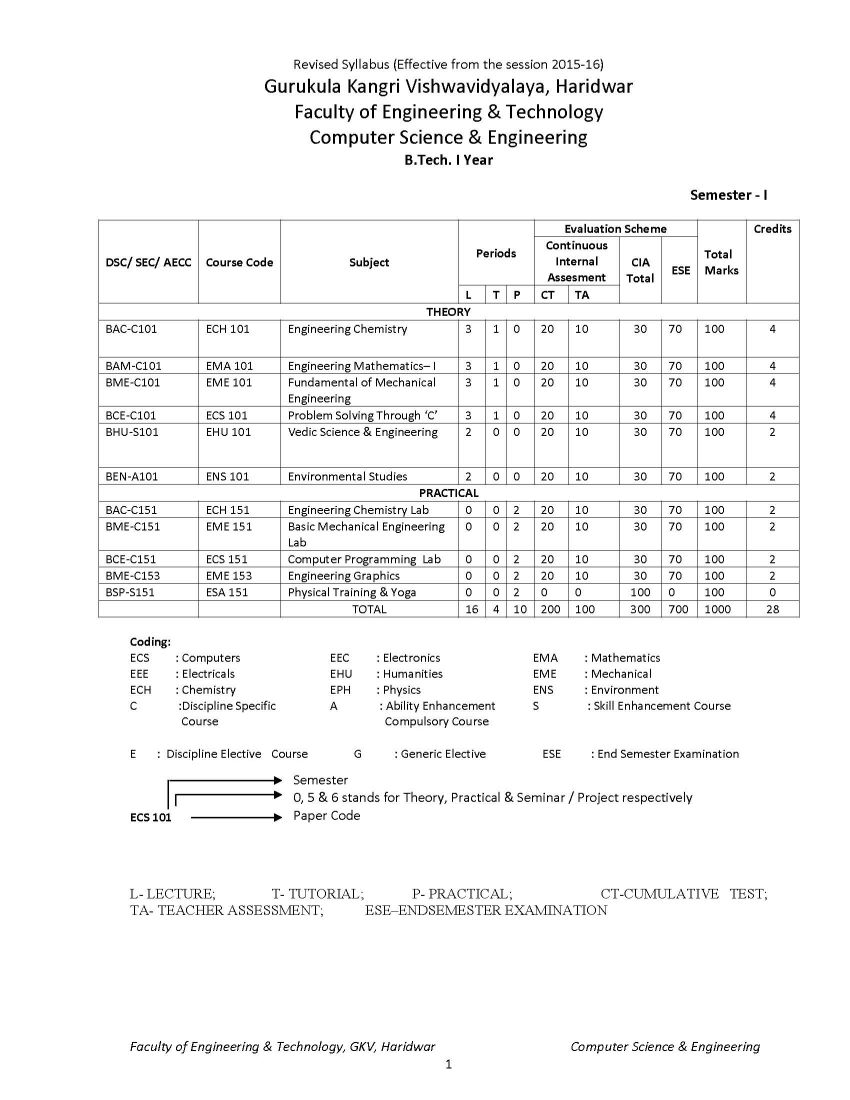 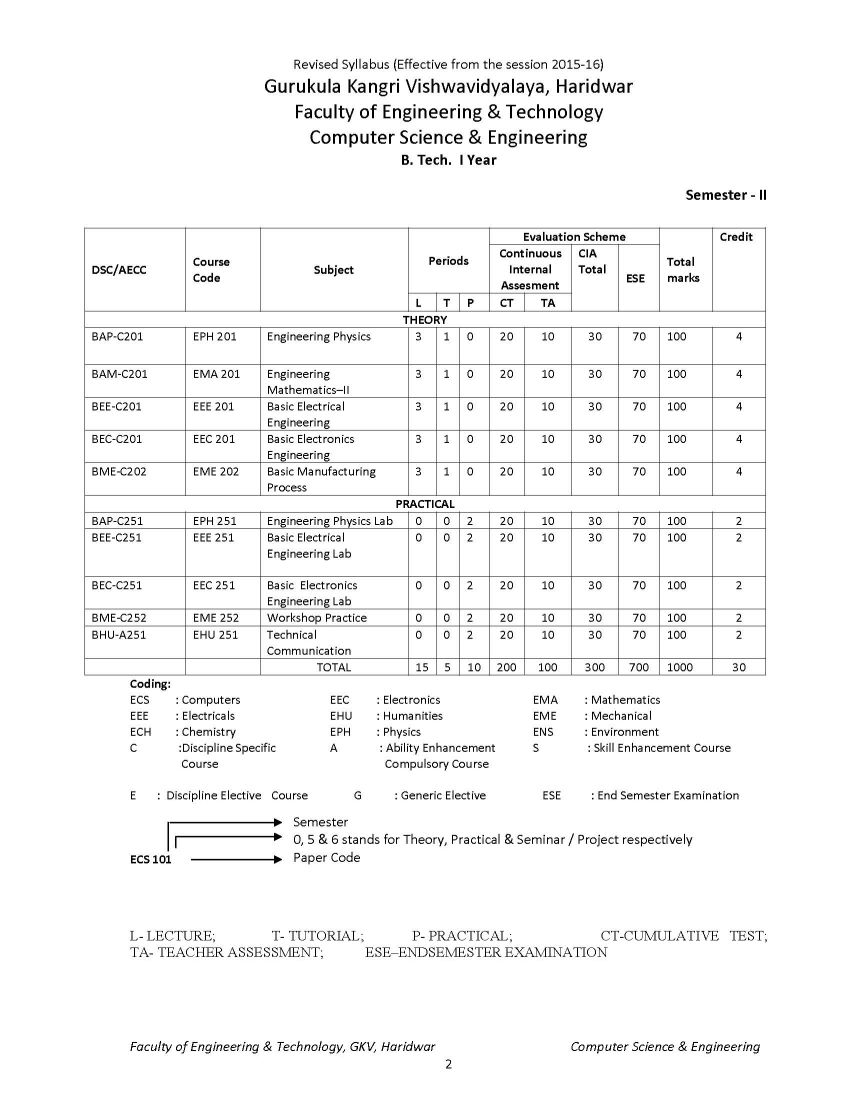 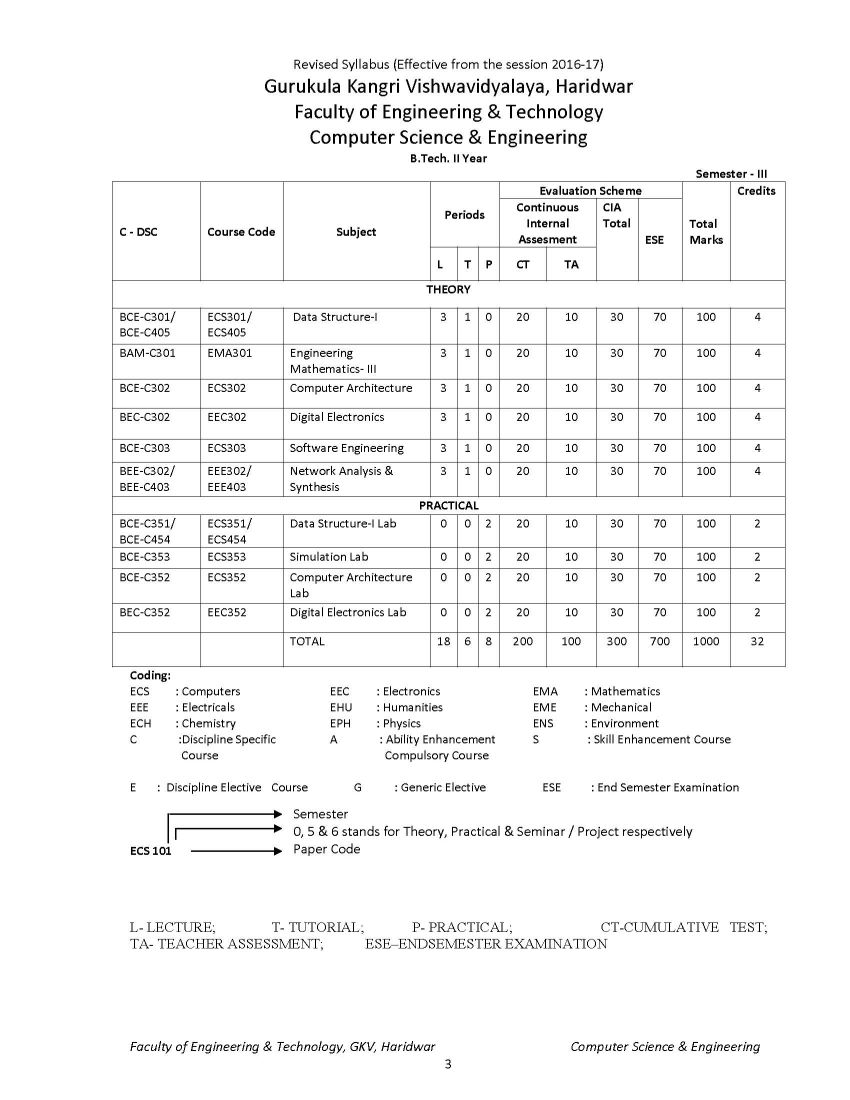 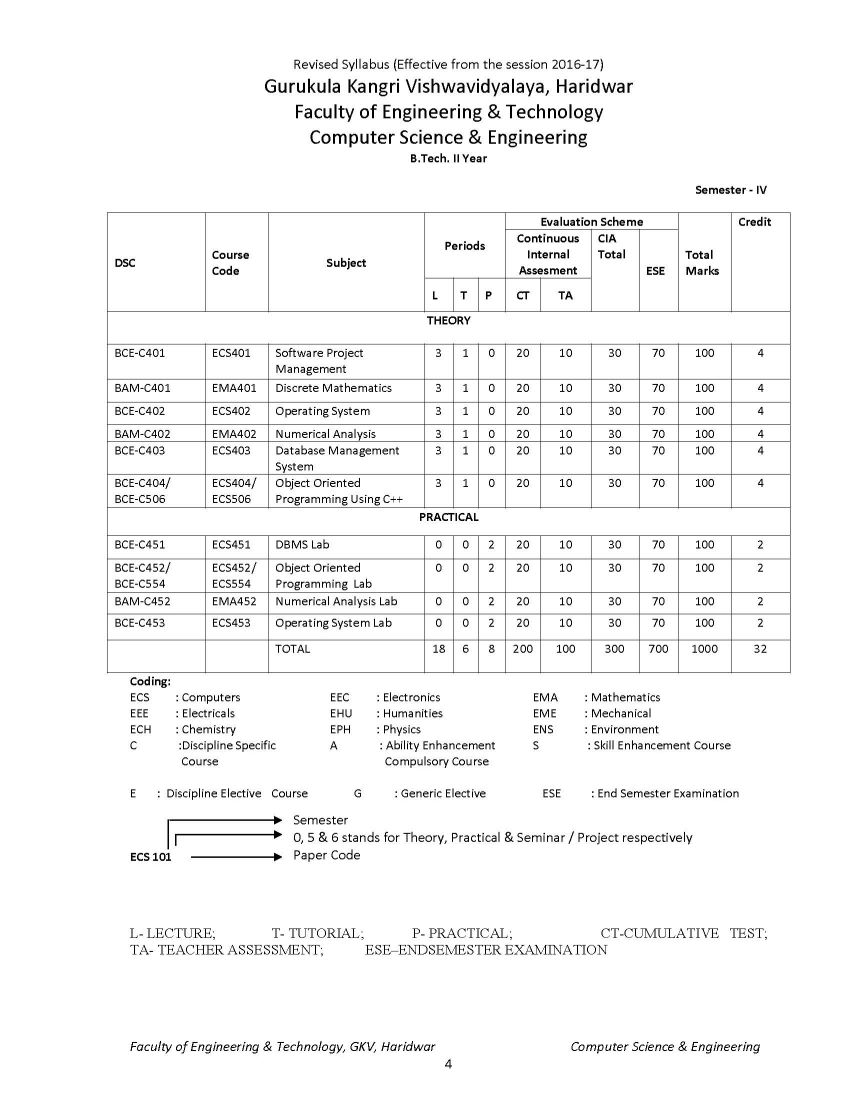 Syllabus B.Tech Computer Science & Engineering Gurukula Kangri Vishwavidyalaya, Haridwar gkv.ac.in/fwd/CSE-CBCS-2015-16.pdf |