|
#3
20th July 2018, 04:20 PM
| |||
| |||
| Re: Eosinophils fsc ssc
As you are looking for information about identification of Eosinophils FSC SSC and Detection and quantitation of eosinophils, so here I am providing following information: Detection and quantitation of eosinophils with other leukocyte subsets in human peripheral blood using the NovoCyte ow cytometer: During hematopoiesis, eosinophils dierentiate from myeloid cells in the bone marrow prior to migration into the blood. They are responsible for combating parasitic infections and also play an essential role in inammatory responses and injury occurring during an allergic response. The traditional method to identify and quantitate eosinophils in inammatory tissues and exudates relies on morphologic criteria and manual counting (i.e. prominent eosinophilic granules will appear as orange-red intracellular structures after H&E staining). However, limitations to this approach result from variability in sample preparation and subjective interpretation of inclusion criteria for inammatory cells based on morphology alone. In this application note, Dr Paul Hutchinson and his team at National University of Singapore collaborated with ACEA Biosciences and utilized a ow cytometry-based assay to identify eosinophils in human blood. This method applies a more rigorous technique for detection and allows for the exact quantitation of the cell populations of interest. Human whole blood (treated with EDTA anti-coagulant) was taken from a normal donor and stained with antibodies against CD4, CD8, and CCR3. Red blood cells were then lysed in BD FACSlyse solution and the sample was washed and xed in 2% paraformaldehyde. Human peripheral blood leukocytes samples were separately acquired using the ACEA NovoCyte ow cytometer as well as a competitor's instrument. Data analysis was performed with ACEAs NovoExpress™ software. We found that the wide dynamic range of detection (7.2 logarithmic decades) provided by the NovoCyte ow cytometer made it easy to identify eosinophils and lymphocytes in the same plot (Fig 1A). In contrast, the limited dynamic range of the competitors instrument restricted our ability to identify these two cell types in the same plot. In fact, adjusting PMT voltages to accurately identify the lymphocyte population resulted in poor resolution of the eosinophils and vice versa. The NovoCyte ow cytometer also allowed us to clearly distinguish monocytes from granulocytes (Fig 1A&B). Absolute cell counts from each of these populations are easily obtained using the NovoExpress software coupled with the highly accurate syringe pump within the NovoCyte system. We then wanted to further verify that we had accurately identied the eosinophils based on their FSC/SSC prole. Since eosinophils are intrinsically autouorescent, we were able to gate on the autouorescent cells in an unstained sample and backgate these to a FSC/SSC dot plot (Fig 2A&B). CCR3 is a chemokine receptor found to be primarily on eosinophils. When comparing autouorescent cells to CD4 and CD8 lymphocytes, the autouorescent cells had much higher levels of CCR3 than both the CD4 and CD8 positive populations (Fig 2C&D). Backgating these populations to a FSC/SSC plot localized the CD4 and CD8 positive cells to where lymphocytes normally reside and the autouorescent/CCR3 positive cells to where we would expect the eosinophils to reside (Fig 2E). This ow cytometer-based assay oers rapid, accurate and unbiased identication and quantitation of eosinophils in human peripheral blood as compared to conventional methods of staining and manual counting. The identification of eosinophils is based on both its FSC/SSC prole and strong intrinsic auto-uorescence in the FITC and PE channels. An additional eosinophil marker, CCR3, was used to further conrm the identity of the auto-uorescent population. Since eosinophils are captured together with lymphocytes, monocytes, and granulocytes, the wide dynamic range oered by the NovoCyte ow cytometer greatly facilitates the resolution of all of these cell types in the same FSC/SSC plot. We were even able to obtain superb separation of the monocyte and granulocyte populations based solely on their scatter proles. Highly accurate absolute cell counts for each of these cell types was also possible, enabling the generation of additional analytic data. Detection and quantitation of eosinophils with other leukocyte subsets in human peripheral blood using the NovoCyte ow cytometer: 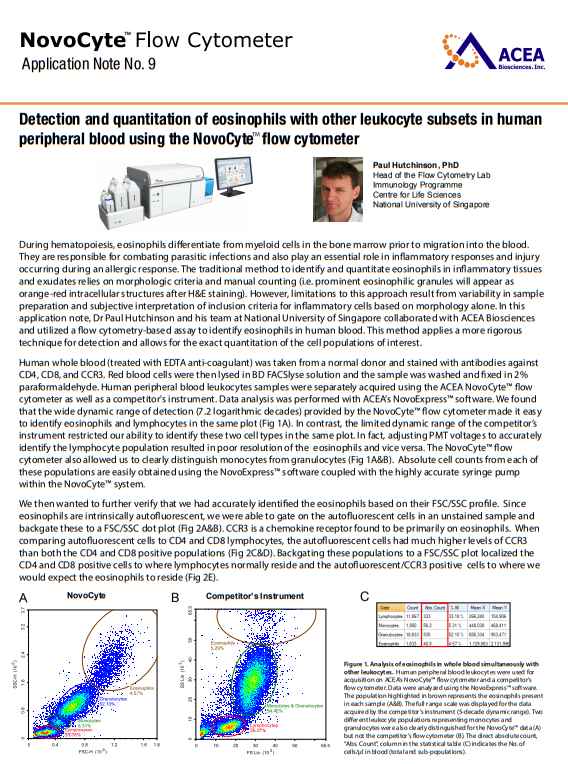 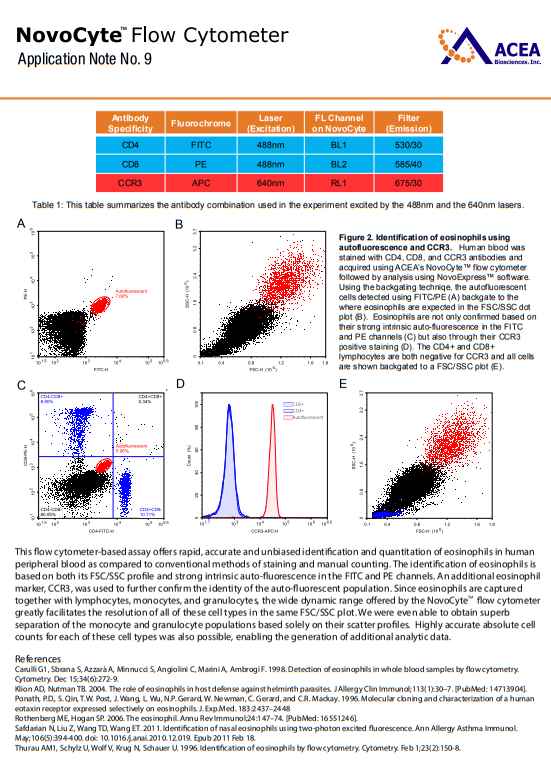 |