|
#2
28th December 2015, 05:24 PM
| |||
| |||
| Re: BSC Syllabus of Kanpur University
As you requires I am providing you syllabus for BSC in Chemistry course offered by Chhatrapati Shahu Ji Maharaj University, Kanpur. BSC in Chemistry Syllabus : First Year : Paper – I Inorganic Chemistry Paper – II Organic Chemistry Paper – III Physical Chemistry Inorganic Chemistry : Unit – I I. Atomic Structure: Idea of de-Broglie matter waves, Heisenberg uncertainty principle, atomic orbitals, Schrödinger wave equation, significance of and 2, quantum numbers, radial and angular wave functions and probability distribution curves, shapes of s, p, d, orbitals, Aufbau and Pauli exclusion principles, Hund's multiplicity rule, Electronic configurations of the elements, effective nuclear charge. II. Periodic Properties: Atomic and ionic radii, ionization energy, electron affinity and electronegativitydefinition, methods of determination or evaluation, trends in periodic table and applications in predicting and explaining the chemical behaviour. Unit – II III. Chemical Bonding: (A) Covalent Bond – Valence bond theory and its limitations, directional characteristics of covalent bond, various types of hybridization and shapes of simple inorganic molecules and ions, valence shall electron pair repulsion (VSEPR) theory to NH3, H3O+, SF4, CIF3, ICl- 2 and H2O, MO theory, homonuclear and heteronuclear (CO and NO) diatomic molecules, multicenter bonding in electron deficient molecules, bond strength and bond energy, percentage ionic character from dipole moment and electro-negativity difference. (B) Ionic Solids – Ionic structures, radius ratio effect and coordination number, limitation of radius ratio rule, lattice defects, semiconductors, lattice energy and Born-Haber cycle, salvation energy and solubility of ionic solids, polarizing power and polarisability of ions, Fajan's rule, Metallic bond-free electron, valence bond and band theories. (C) Weak Interactions – Hydrogen bonding, Vander Waals forces. Unit – III IV. s-Block Elements: Comparative study, diagonal relationship, salient features of hydrides, solvation and complexation tendencies includeing their function in biosystems, an introduction to alkyls and aryls. V. Chemistry of Noble Gasses: Chemical properties of the noble gases, chemistry of xenon, structure and bonding in xenon compounds. Unit – IV VI. p-Block Elements: Comparative study (including diagonal relationship) of groups 13-17 elements, compounds like hydrides, oxides, oxyacids and halides of group 13-16, hydrides of boron-diborane and higher boranes, borazine, borohydrides, fullerenes, carbides, fluorocarbons, silicates (structural principle), tetrasulphur tetra nitride, basic properties of halogens, interhalogens and polyhalides. 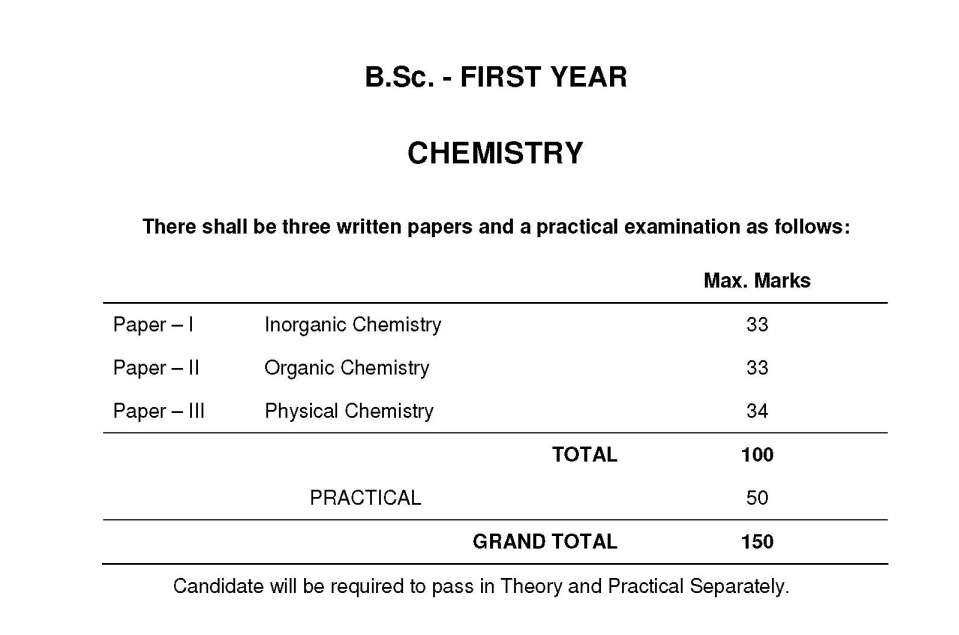     Here is the attachment. Address: Chhatrapati Shahu Ji Maharaj University Aligarh-Kanpur Road, Indira Nagar Kanpur, Uttar Pradesh 208024 Last edited by sumit; 12th December 2019 at 03:08 PM. |