|
#2
14th August 2014, 12:44 PM
| |||
| |||
| Re: SET Chemistry Entrance Exam Previous Paper
Here I am giving you question paper for Maharashtra State Eligibility Test for Lectureship (SET) Chemistry Entrance Exam in a PDF file attached with it so you can get it easily. 1. Which of the folloiwng is not a Lewis base ? (A) CN– (B) C2H5OH (C) AlCl3 (D) (CH3)3N 2. The bond orders of the + 2 2 O , O and 2 O− are respectively : (A) 2.5, 2.0, 1.5 (B) 2.0, 2.5, 1.5 (C) 2.0, 1.5, 2.5 (D) 2.0, 2.5, 2.5 3. For the concentration cell Ag|Ag+ (aq., 0.01 mol dm–3) || Ag+ (aq, 0.1 mol dm–3)|Ag the EMF of the cell, E at a temperature T equals : (A) RT 2.303 F (B) RT 2.303 F − (C) + 0 Ag , Ag RT E 2.303 F + (D) + 0 Ag , Ag RT E 2.303 F − 4. The pH of a 1.0 × 10–3 mol dm–3 solution of a weak acid HA is 4.0. The dissociation constant of the acid is : (A) 1.0 × 10–3 (B) 1.0 × 10–4 (C) 1.0 × 10–5 (D) 2.0 × 10–5 5. The point groups of 1, 2-dichloro- benzene, 1, 3-dichlorobenzene and 1, 4-dichlorobenzene are respectively : (A) C2V, C2V, D2h (B) D2h, D2h, C2V (C) C2h, C2h, D2h (D) C2V, D2h, C2V 6. The term symbol for a particular state of an atom is 3D3. The values of L, S and J for this term are respectively : (A) 3, 1, 3 (B) 2, 1, 3 (C) 2, 0, 3 (D) 3, 2, 3 7. Given that the standard potentials of the Cu2+/Cu and Cu+/Cu couples are + 0.340 V and 0.522 V respectively, the standard potential of Cu2+/Cu+ couple is : (A) 0.182 V (B) 0.862 V (C) + 0.158 V (D) – 0.158 V 8. Which of the following molecules does not possess a centre of symmetry ? (A) trans-dichloroethene (B) naphthalene (C) eclipsed ethane (D) staggered ethane 9. An ideal gas in a thermally insulated vessel expands, quickly against vacuum. For this process : (A) the temperature remains constant (B) a finite, non-zero amount of work is done by the gas (C) heat flows into the gas (D) intermolecular interactions decrease upon expansion 10. Standard enthalpies of formation (in kJ mol–1) of four compounds A, B, C and D are – 200, – 50, + 10 and – 100 respectively. For the reaction : A + 2C = 3B + 4D. The standard enthalpy of reaction, in kJ mol–1, is : (A) 40 (B) 0 (C) –340 (D) –370 SET Chemistry Entrance Exam Paper 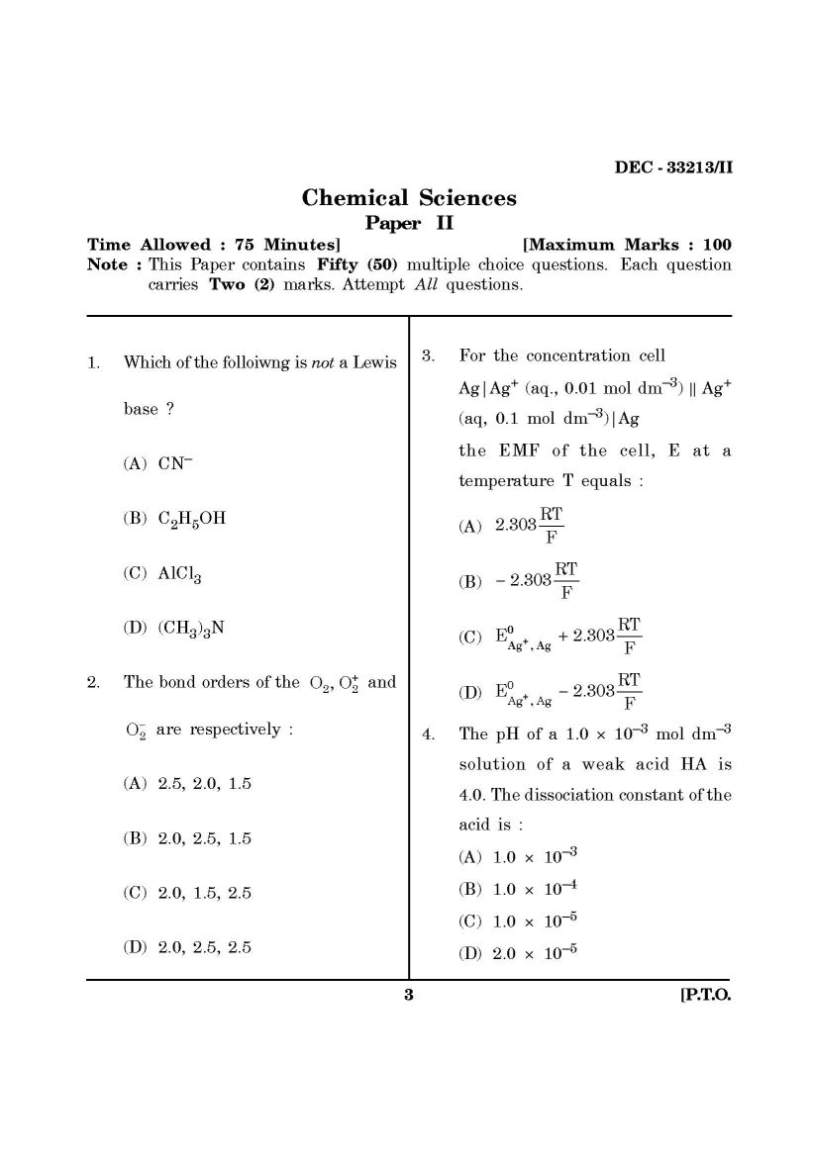  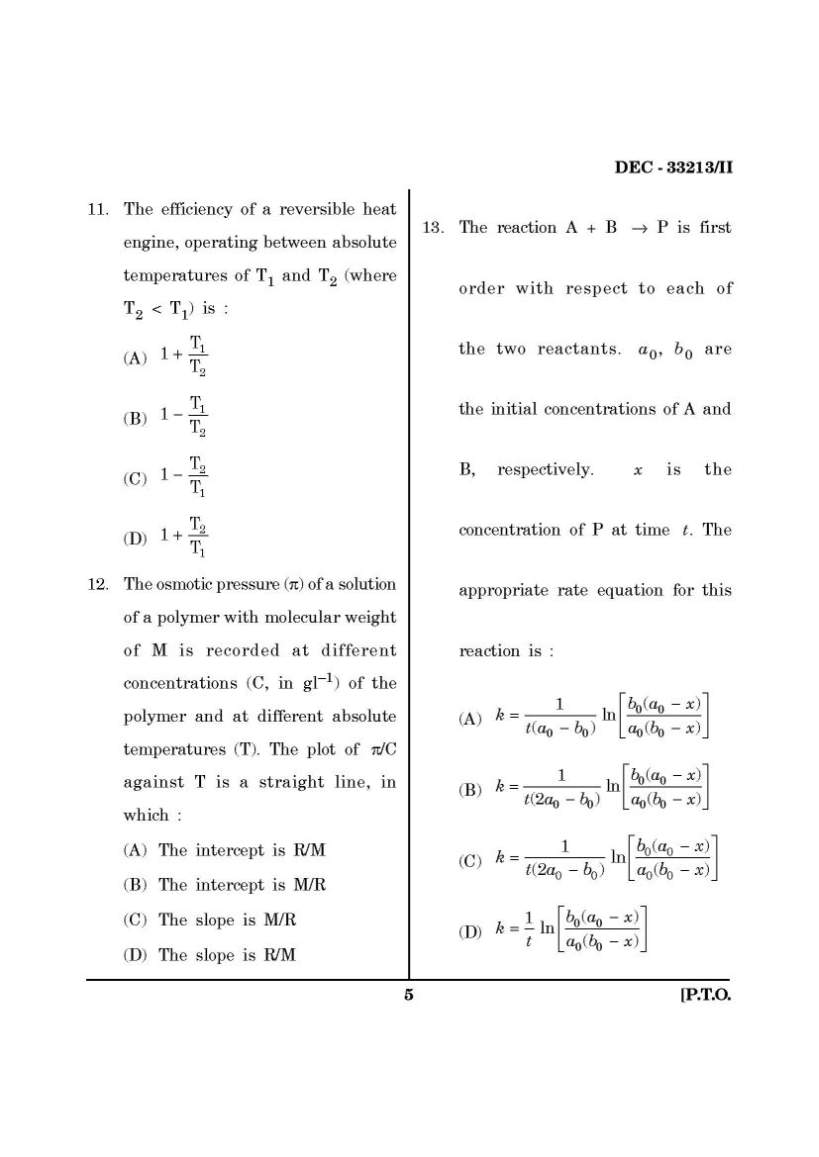 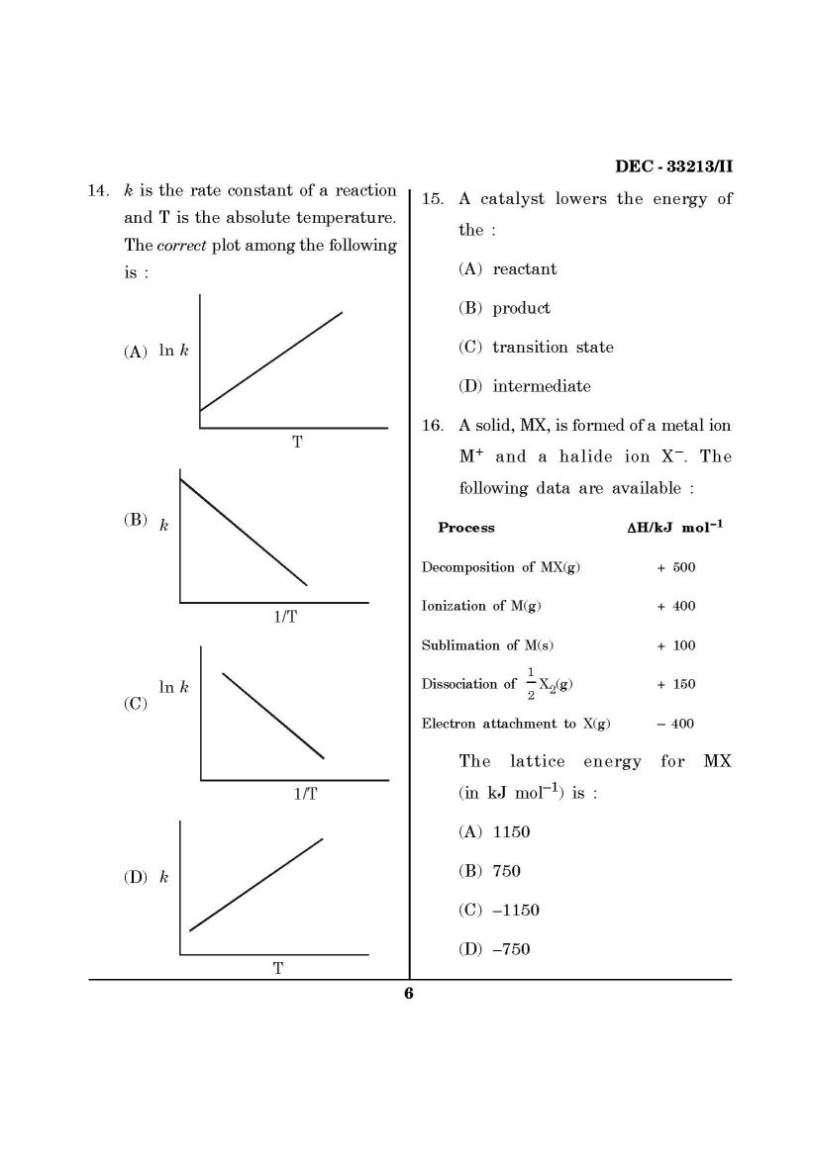 |