|
#2
19th November 2015, 11:34 AM
| |||
| |||
| Re: SECL Lewis Structure
Feel free buddy I will help you here to get the information about the SECL Lewis Structure so that you can have idea about it and solve it easily. Write the total number of valence electrons in the box containing the formula of the molecule. Draw Lewis Structures for each of the following molecules. Check to be sure to have the correct number of electrons drawn in for each structure and that each atom has the correct number of electrons around it. Molecule Lewis Structure Molecule O 2 N 2 CS 2 NF 3 H 2 S S is in center POCl 3 SO 2 PCl 5 SbCl 5 2- SF 6 SF 4 XeF 4 IF 5 XeF 2 CH 4 O 3 SECL Lewis Structure  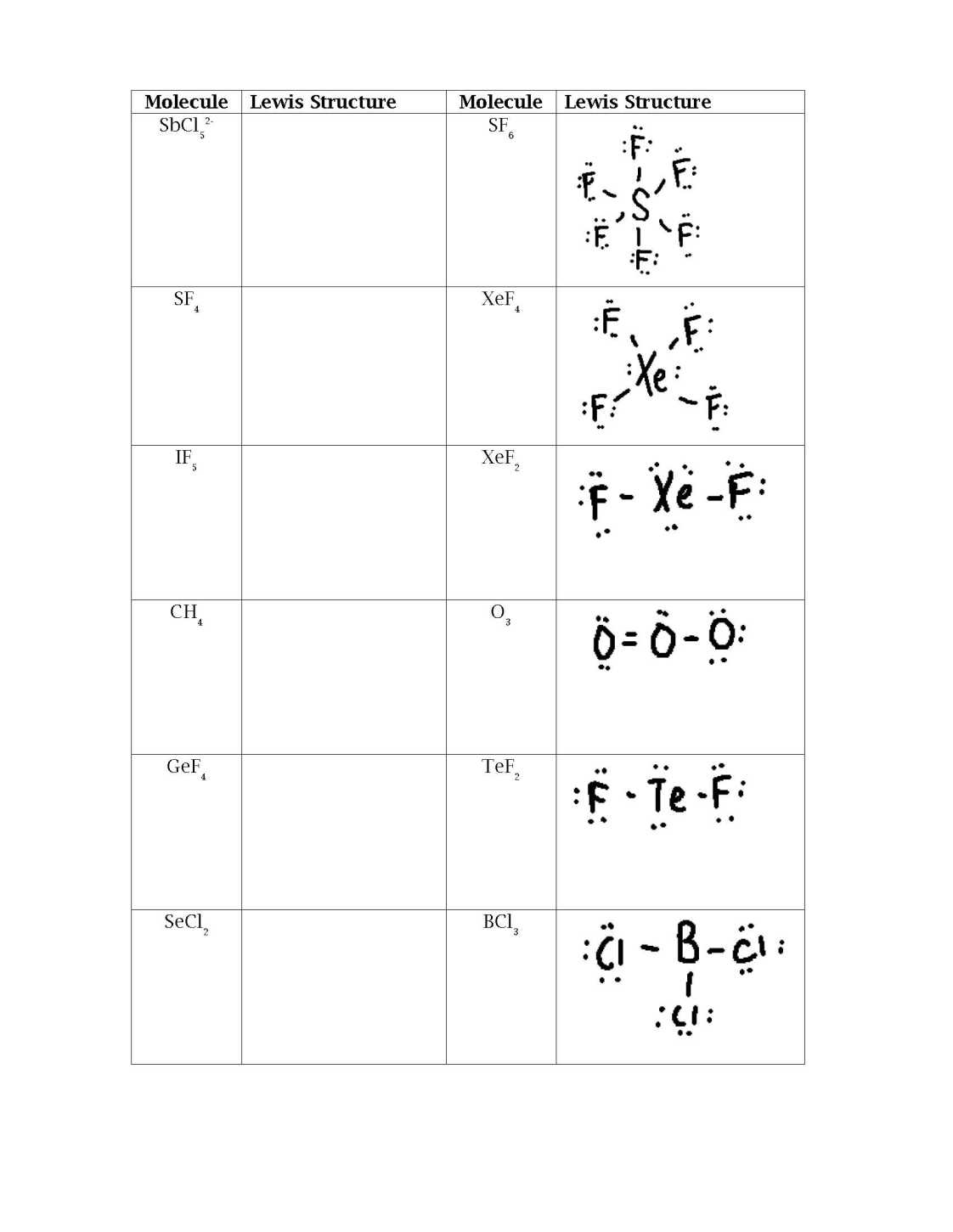 |