|
#2
28th September 2016, 04:37 PM
| |||
| |||
| Re: PI Point Amino Acids
The isoelectronic point or isoionic point is the pH at which the amino corrosive does not relocate in an electric field. This implies it is the pH at which the amino corrosive is impartial, i.e. the zwitterion structure is prevailing. The pI is given by the normal of the pKas that include the zwitterion, i.e. that give the limits to its presence. The pKa values and the isoelectronic point, pI, are given underneath for the 20 α-amino acids. pKa1= α-carboxyl gathering, pKa2 = α-ammonium particle, and pKa3 = side chain bunch. 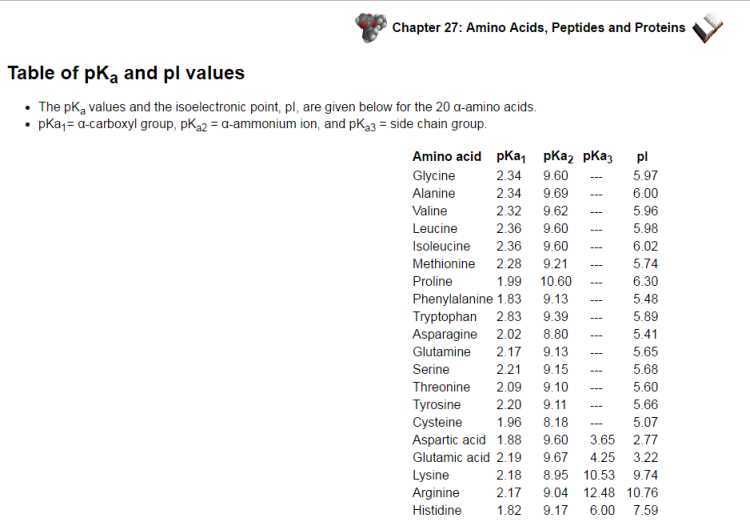 There are 3 cases to consider: unbiased side chains These amino acids are portrayed by two pKas : pKa1 and pKa2 for the carboxylic corrosive and the amine separately. The isoelectronic point will be somewhere between, or the normal of, these two pKas, i.e. pI = 1/2 (pKa1 + pKa2). This is most promptly refreshing when you understand that at extremely acidic pH (beneath pKa1) the amino corrosive will have a general +ve charge and at exceptionally essential pH (above pKa2 ) the amino corrosive will have an in general - ve charge. For the least difficult amino corrosive, glycine, pKa1= 2.34 and pKa2 = 9.6, pI = 5.97. The other two cases present other ionisable gatherings in the side chain "R" depicted by a third corrosive separation consistent, pKa3 acidic side chains The pI will be at a lower pH in light of the fact that the acidic side chain presents an "additional" negative charge. So the nonpartisan structure exists under more acidic conditions when the additional - ve has been killed. For instance, for aspartic corrosive demonstrated as follows, the nonpartisan structure is overwhelming between pH 1.88 and 3.65, pI is somewhere between these two qualities, i.e. pI = 1/2 (pKa1 + pKa3), so pI = 2.77. essential side chains The pI will be at a higher pH in light of the fact that the essential side chain presents an "additional" positive charge. So the impartial structure exists under more fundamental conditions when the additional +ve has been killed. For instance, for histidine, which was talked about on the past page, the nonpartisan structure is prevailing between pH 6.00 and 9.17, pI is somewhere between these two qualities, i.e. pI = 1/2 (pKa2 + pKa3), so pI = 7.59. |