|
#2
24th November 2014, 01:53 PM
| |||
| |||
| Re: PG TRB Chemistry Question Papers
The answer key of the TRB PGT Chemistry Question Paper conducted on 21.7.2013 is as follows: PG TRB Chemistry Answer Key  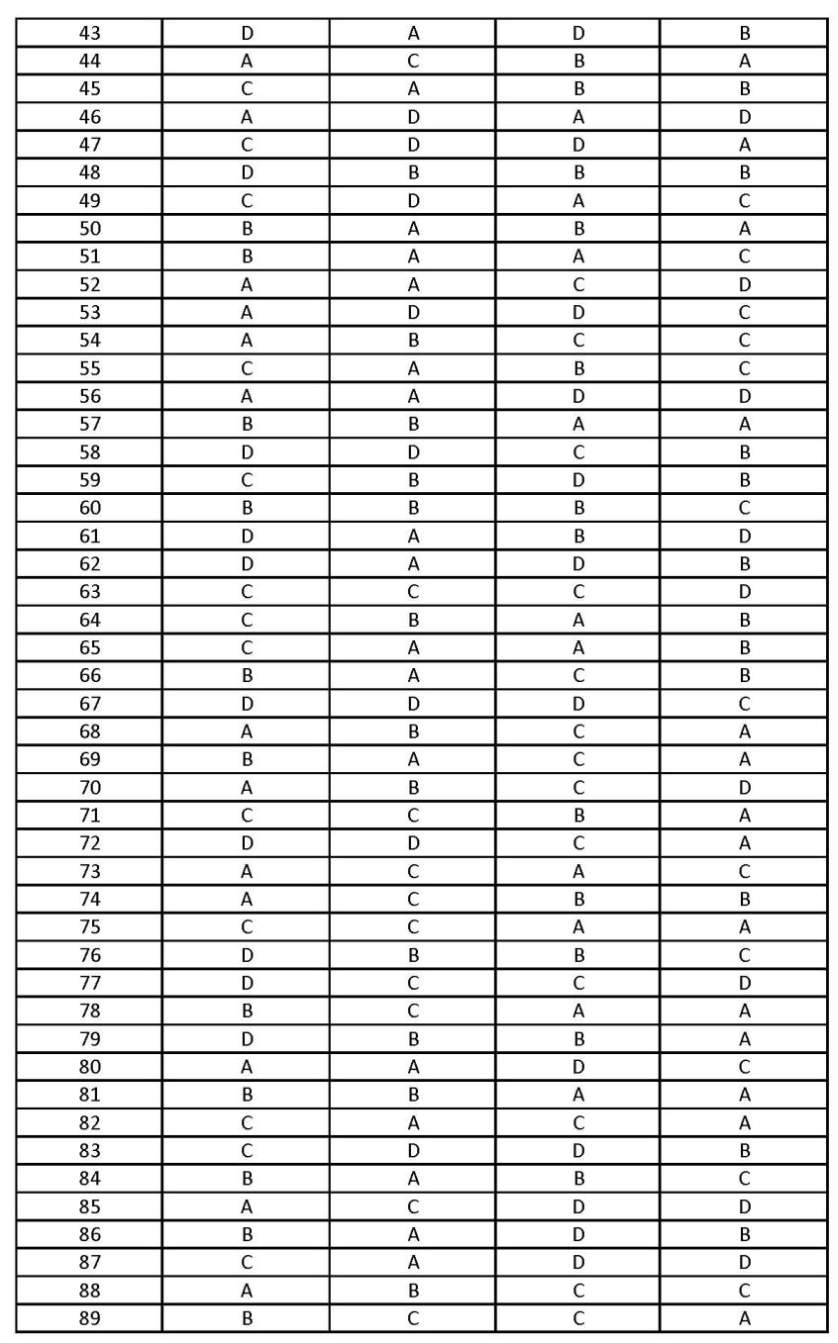   Syllabus PG TRB Chemistry Unit-I Periodic properties – Atomic radius – ionic radius, ionization potential, electron affinity and electronegativity – Their significance in chemical bonding. VB theory, MO theory – applications – Comparision of VB and MO theories – VSEPR theory – Bond order – Bond energy – Bond length Bond polarity – Partial ionic character of bonds – The concept of multi-centre bond – Electron deficient compounds – Hydrogen bond – Its influences. Non aqueous solvents – A general study of typical reactions in non aquieous media – comparison with reactions in aqueous media. Solid state chemistry – Ionic bonding – Lattice energy – Born equation – Born Haber cycle – Radius ratio rule – Born Meyer equation – Kapustinski’s Modification – energetics of the dissolution of ionic compounds in polar solvents – different types of electostatic interactions. Structural aspects of solids – Fourier synthesis and analysis structure factors – scattering factors – Spinels and Inverse spinels – defects in stoinchiometric and Non stoichiometric crystals. Electrical properties of solids – Band theory semi conductors – Junction devices – Super conductivity – Ionic conductivity – Optical properties of solids – Lasers and phosphors – Photovoltaic effect – solar energy. Magnetic properties of solids – Different types – dia, para, ferro, antiferro and ferri Magnetism – Magnetic hysteresis. Unit II Co-ordination chemistry – Methods of preparation of complexes – isomerism in complexes – applications of complex formation in analytical chemistry – complexes and their stability chelate effect Stability constants – Their determination – complexes of Metals in different oxidation states and their stability. Optical activity and concept of chirality – Different kinds of opticalloy active compounds – configuration – Foscher, sawhorse and Newman projections – Absolute configuration R and S Notations – Methods with more than one chiral center – Asymmetric synthesis – optical purity. Geometrical isomerism resulting from double bonds – The E.Z. system of nemenclature – Geometrical isomerism of monocyclic compounds and fused ring systems – Sterospecific and stereo selective reactions with examples. Confermational analysis – conformation and reactivity in acylic and cyclo – hexane systems – conformation of decalins, cyclohexane and cyclohexanone. Unit III Organic reaction mechanisms – General methods of investigating reaction mechanisms – kinetic and non-kinetic methods – different types of reaction intermediates. Aliphatic nucleophilic substituion SN1, SN2 and SNi reactions – substitution at vinylic and benzylic carbon – stereo chemistry of nucleophilic reaction – solvents and substituent effects – Nucleophilicity Neighboring group participation. Addition to double and triple bonds – Mechanism Hydration – Hydroboration – Hydroxiylation – epoxidation. Elimination reactions E1, E2, E1cB Mechanism – Orientation effects in elimination reactions – stereo chemistry of elimination reactors - dehydration of alcohols – dehydro halogenation – cope elemination. Heterocyclics – synthesis and reactivity of furan, thiophene, pyrrole pyridine, quinoline, isoquinoline, Indole, flavenes, and anthocyanins – skraup synthesis – Fischer indole synthesis. The chemistry ;of natural products structure elucidations and Biogenesis of the following: Alkaloids : Reticulene, Reserpine, Morphine Terpenoids : Zingiberene, Squalene, Lanosteroal Steroids : Cholesterol, Oestrone, Progresterone Carbohydrates: Maltose, Starch, Cellulose (biogenis not expected) Structure and functions of biopolymer such as proteins and Nucleic acids – Primary, Secondary and tertiary structures of proteins – Mechanism of Enzyme action – DNA and RNA. Unit IV The old quantum theory – Inadequacy of classical mechanics – Failure of classical mechanics – success of quantum hypothesis explaining black body radiation – Photo electric effect – the hydrogen spectrum – Bohr’s explanation of hydrogen spectrum – Failure of Bohr’s model. De broglie’s postulates of Matter waves – experimental observation of matter waves – Heisenberg’s uncertainly principle – wave particle dualism – Davisson, Garmer experiments – Postulates of quantum mechanics – Time dependent schrodinger equation – Needs of an acceptable wave function – Physical significance of Psi function. Operators in quantum mechanics. Operator algebra – Linear and Hermitian operators –m Eigen functions and Eigen values – Hamiltonian operators – Angular momentum. Application of schrodinger equation – particle in one and three dimensional boxes – quantum mechanical results for a simple harmonic oscillaltor and rigid rotator - approxination methods – perturbation methods – variation method – VB and MO methods. Symmetry elements and symmetry operations – Point groups – representation of groups reducible and irreducible representations characters tables – Orthogonality theorem and its consequences. Symmetry selection rule for IR and Ramanspectra – Systematic procedure for determining symmetries of normal modes of vibration – symmetry applied to MO theory and orbital hybridization. Unit V Thermodynamic equations of state – closed and open systems – partial molal quantities – chemical potential with temperature and pressure – third law of thermodynamics. Fugacity – methods of determination – activity and activity co-efficient – standard states for gases, liquids – solids and solutions – mean activity co-efficients of electrolytes. Maxwell’s distribution of molecular velocities – derivation of expression for average, most probable and rcot mean square velocities – Microstates Macrostates – partial functions – Sackur tetrode equation – statistical approach to the third law of Thermodynamics – Maxwell Boltzmann – Bose Einstein and Fermi Dirace statistics – Heat capacities of solids – Einstein and Debye Models Low temperature – Negative absolute temperature. Chemical equilibrium – thermodynamic derivation of equilibrium constant – standard free energy – calculations. Phase equilibrium – thermodynamic derivation of phase rule application of phase rule – three component systems. Chromotography – column, paper, thinlayer, gas-liquid, High pressure liquid chromatography HPLC principle and applications. Thermal analysis – different thermal analysis (DTA) – Principle and applications – thermogravimetric analysis (TGA) Principle and application. Chemical crystallography – Diffraction methods – X ray Neutron, electron diffraction methods. Principle and applications. Polarimetry – Circular ichroism – Optical Rotatory dispersion (ORD) Principle and applications. Unit VI Nuclear – chemistry – Nuclear nadii spin and moments – Nuclear structure – Nuclear forces – Nuclear stability – Nuclear modes – Modes of Radioactivity decay. Nuclear isomerisation Nuclear Reaction Energy – Coulomb barrier cross section – excitation function – Radiactive Equiliberia – Types of Neclear reactions – Nuclear fision Nuclear Reactors – Atomic Power Project in India – Radiation hazards – Radiation desimetry – Nuclear fusion – Stellar Energy. Application of Radioactivity – Tracer Techniques – Neutron - Activation analysis – Isotope Dilution Analysis – Interaction of radiation with matter – Range of alpha and beta particles – Absorption co-efficient,. Orgnometallic compounds – Metallecences – Arene complexes – Nonaromatie olefins and acetylenes complexes – catalysis by organometallic compounds Wilkinson’s catalyst – Oxoprocess – wecker process – Ziegler – Natta catalysis. Inorganic photo chemistry – Photochemical reactions of coordination and organ metallic compounds – Properties of excited states – charge transfer photo Oxidation, photo reduction, photo substitution, photo isomerisation - Photo chemical conversion – Solar energy. Unit VII Term symbols and term states – Dn - ions energy levels – Diagrams weakfield and strong field and strong field concepts – spin orbit coupling – The Nephelanxetic effect charge transfer spectra – Applications of UV, IR, NMR, BSR and mossbaver spectroscopy techniques in the study of co-ordination chemistry. Magnetic interactions – Magnetic susceptibilities determination – application in co-ordination chemistry. Application of VB, MO, CF and LF theories in co-ordination chemistry – Group theoretical approach – splitting of d-orbitals – spectro-chemical series – concept of weak and strong fields – Thermodynamic and chemical effect of d-orbitals splitting – Jahn Teller distortion. Nuclear Magnetic Resonance Spectroscopy – Theory – Study of PMR – chemical shift – Type of shielding – Spin-spin coupling spin decoupling – spplications to simple natural products. Electron spin resonance spectro scopy – paramagnetism – Nuclear hyperfine structure – Hyperfine coupling. Unit VIII Huckel’s rule and concept of aromaticity – aromaticity of Benzenoid – Nonbenzenoid aromatics. The annulenes - Aromaticity in charged rings and fused ring systems. Aromatic electrophilic substitution – Mechanism and reactivity, Typical reactions to include diazonium coupling – Halognation, sulphonation. Friedal craft alkylation and acylation. Aromatic Nucleophilic substitution – Benzyne mechanism – Examples. Oxidation – Reduction reactions – Mechanisms – selectivity in oxidations and reductions. Molecular rearrangements – Rearrangements with Carbon to Nitrogen, Carton to Oxygen and Carbon migrations. Curtivs, Lossen, Schmitts Baeyer – Villiger, Pinacol – Pinacolene, Benzoil – Benzilicacid, Benzidine, Favorski and fries rearrangements – sigmatropic rearragements – claisen and cope. Pericyclic reactions, selection rules – orbital symmetry – Electrocyclic reactions – cycle additions sigmatropic reactions. Modern synthetic reactions – Diels alder reaction witting reactions – stork Enamine reactions – Mannich reactions, Birch reductions. Unit IX Theories of reaction rates – simple collision theory – absolute reaction rate theory (ARRT) – Reaction co-ordinate – Potential energy surfaces. Hammett – Taft equation – Hammett acidity function – Acid base catalysis Bronsted relation Enzyme catalysis – Michaelis Menton Law – influence of PH and temperature. Surface phenomenon – Heterogeneous catalysis – Absorption isotherms. Electrolytic conductance – applications – solubility product – Interionic attraction theory – Debye – Huckel – Onsager equation – equivalent conductivity of electro lytes. Electro potentials – Electrochemical cells – electrode – electrolyte interface – electrical double layer electro capillary phenomena – electro kinetic Phenomena – Membrane potential – Polarisation – over potential – Polarography – concentration polarization – electro chemical polarization – sutler – Volmer equation. Unit X Theory and applications of the following spectroscopic methods; electonics spectra-UV-Visible spectra – IR spectra – Raman spectra – Laser – Raman spectra – NMR – WCR- ESR Spectra – Mossbaver spectro scopy – photoelectron spectro scopy – Poly merisation reactions – Mechanism – sterochemical aspects. Types of polymers – organic and inorganic polymers – preparation – properties – structure – polystyrene – Polyvinylchloride – Polyesters – Nylon – Phenol resin – amino resins – epoxy resins. Phosphonitrilic compounds – silicons – Borazines applications of polymers. |