|
#2
11th June 2015, 04:21 PM
| |||
| |||
| Re: Mumbai University Chemistry
As you are looking for the University of Mumbai T. Y.B.Sc. Chemistry syllabus , here I am providing same for you . 1.1 Colligative Properties of Dilute Solutions (8L) 1.1.1 Dilute solution, colligate properties, Raoult’s law, relative lowering of vapour pressure. 1.1.2 Elevation in boiling point of a solution, thermodynamic derivation relating elevation in the boiling point of a solution and the molar mass of the non-volatile solute. 1.1.3 Depression in freezing point of a solution, thermodynamic derivation relating the depression in the freezing point of a solution and the molar mass of the non-volatile solute. 1.1.4 Osmotic pressure, van’t Hoff’s equation for osmotic pressure, (derivation is expected) and determination of molar mass of the solute. Abnormal molar masses of solutes and van’t Hoff factor (calculation of Degree of Association and Degree of Dissociation.) 1.2 Phase Rule (7L) 1.2.1 Gibb’s phase rule and terms involved in the equation. 1.2.2 Application of phase rule to ONE component systems (i) water system, (ii) sulphur system 1.2.3 Application of phase rule to TWO component systems, condensed systems, condensed phase rule, eutectic systems (Lead-Silver system), desilverisation of lead. 1.2.4 Introduction to three component system, explanation of phase diagram for three liquids forming one immiscible pair. II 2.1 Surface Chemistry & Catalysis 2.1.1 Adsorption: Physical and Chemical Adsorption, types of adsorption isotherms . Langmuir’s adsorption isotherm (Postulates and derivation expected). B.E.T. equation for multilayer adsorption, (derivation not expected). significance of the terms involved in the equation is expected.),determination of surface area of an adsorbent using B.E.T. equation. Numericals on surface area determination are expected. 2.1.2 Catalysis: Homogeneous and heterogeneous catalysis, catalytic activity and selectivity, promoters, inhibitors, catalyst poisoning and deactivation, 2.1.3 Acid-Base catalysis, mechanism and kinetics of acid-base catalyzed reactions, effect of pH on acid-base catalyzed reactions. Mechanism andkinetics of enzyme catalyzed reaction (Michaelis-Menten equation). 2.2 Colloids (6L) 2.2.1 Introduction to colloidal state of matter. 2.2.2 Origin of charge on colloidalparticles. Concept of electrical double layer, zeta potential, Helmholtz and Stern model, Electro-kinetic phenomena:1.Electrophoresis,2.Electrophoresis ,3. Streaming potential 4. Sedimentation potential . 2.2.3 Colloidal electrolytes. 2.2.4 Donnan Membrane Equilibrium. 2.2.5 Surfactants, micelle formation, applications of surfactants in detergents, food industry, in pesticide formulations. University of Mumbai T. Y.B.Sc. Chemistry Syllabus 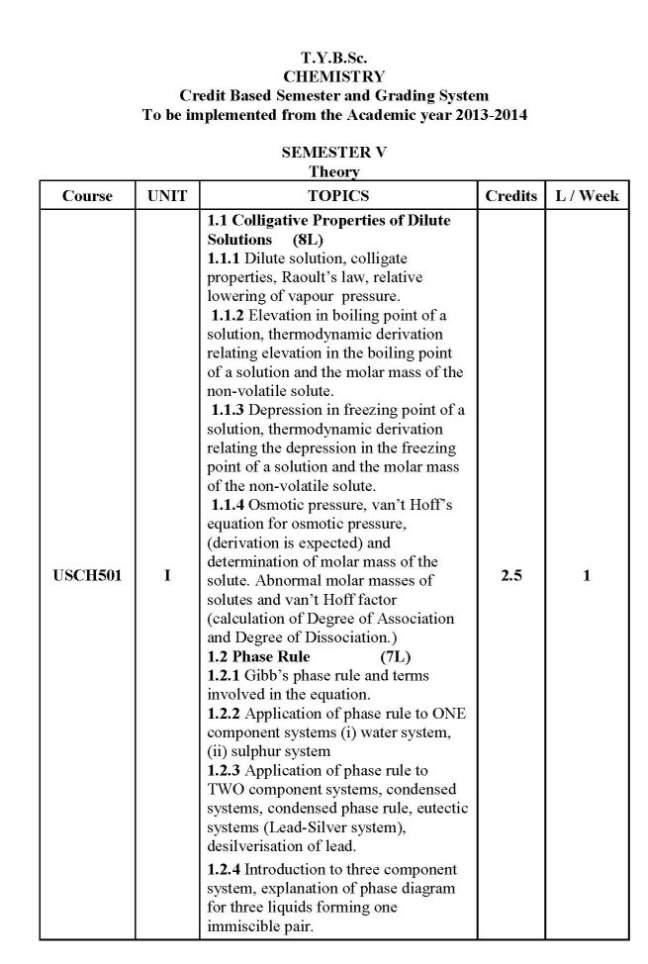 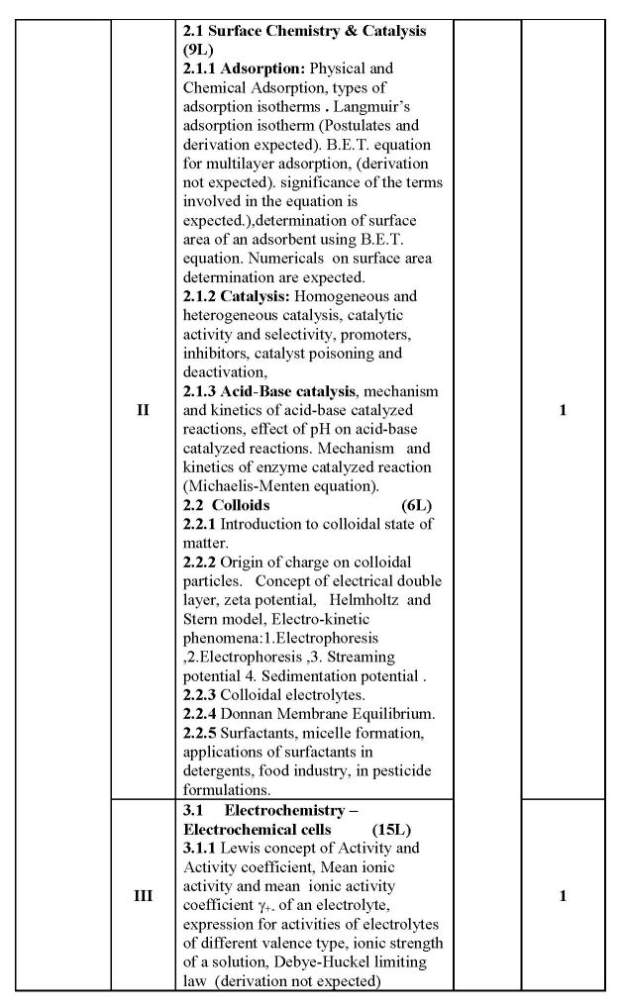 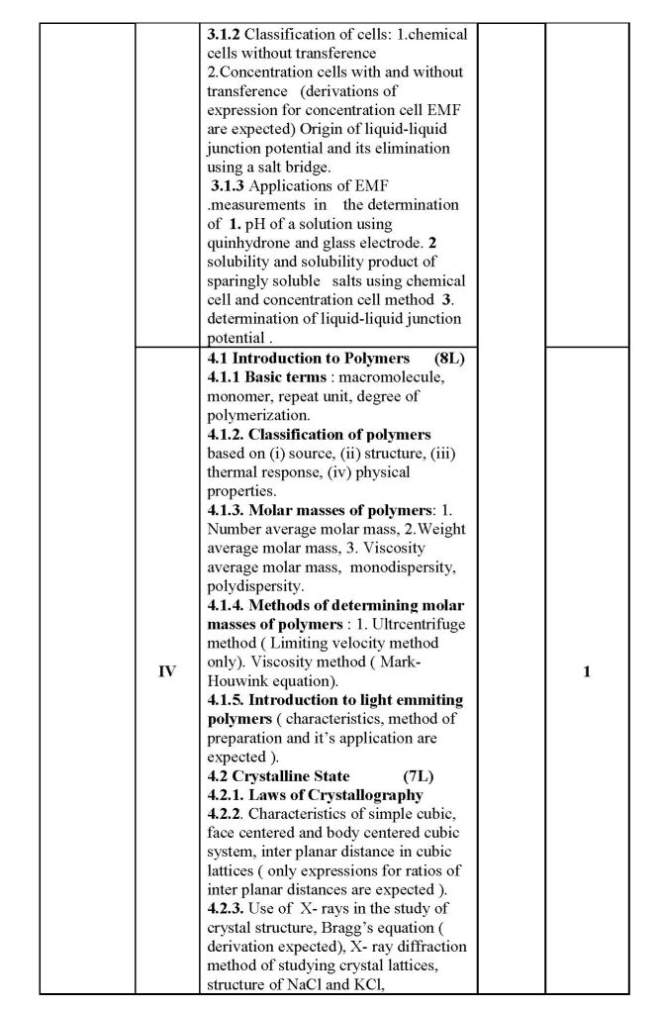 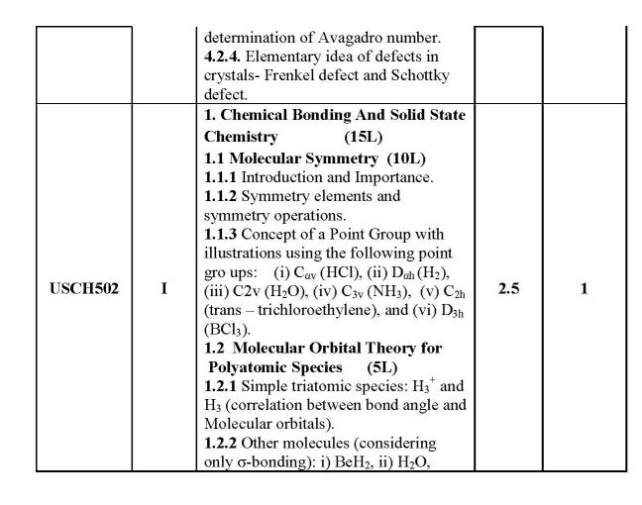 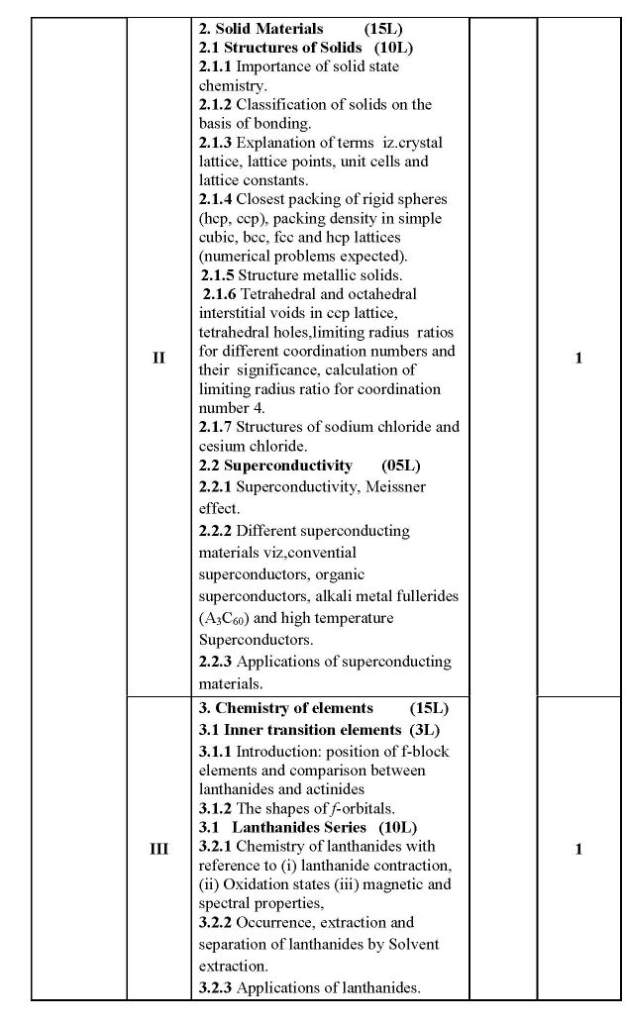 |