|
#2
18th May 2016, 09:04 AM
| |||
| |||
| Re: KEAM Sample Question Papers
As you have asked for the KEAM Engineering sample question papers, I am providing you with it, check below for the details, For an irreversible reaction A ----> 2B, the rate is increased by four times when the concentration of A is doubled. The incorrect statement about this reaction is: 1) It is a second order reaction. 2) Half life is independent of initial concentration of A. 3) The unit of the specific rate, k is L mol-1 s-1. 4) Half life is inversely related to the initial concentration of A. The correct order of ∠FMF bond angles (where M is the central atom) in NSF3, SiF4 and POF3 is: 1) NSF3 > SiF4 > POF3 2) SiF4 > NSF3 > POF3 3) NSF3 > POF3 > SiF4 4) SiF4 > POF3 > NSF3 The outer electronic configuration of metal, M in a diamagnetic complex with the formula, M(NH3)(EtNH2)BrCl is (n-1)d8. It does not give any precipitate with silver nitrate. The correct statement about this complex among the following is: 1) Exhibits optical activity. 2) The metal, M undergoes sp3 hybridization. 3) Can show geometrical isomerism. 4) Its aqueous solution exhibits electrical conductivity. KEAM Engineering sample paper 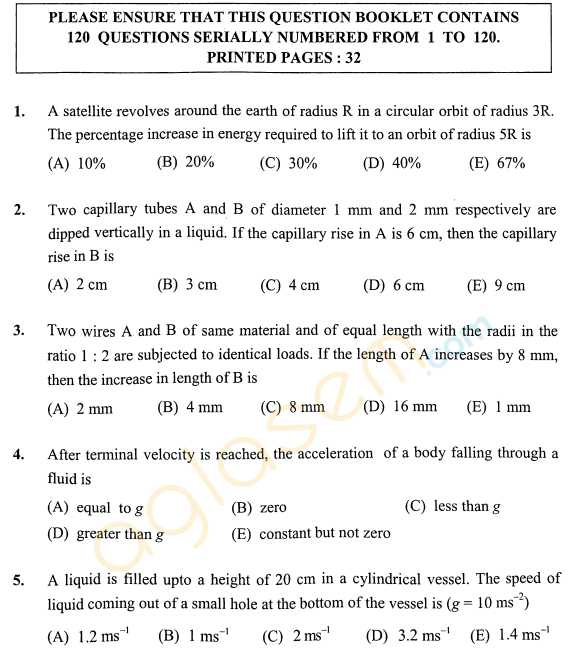 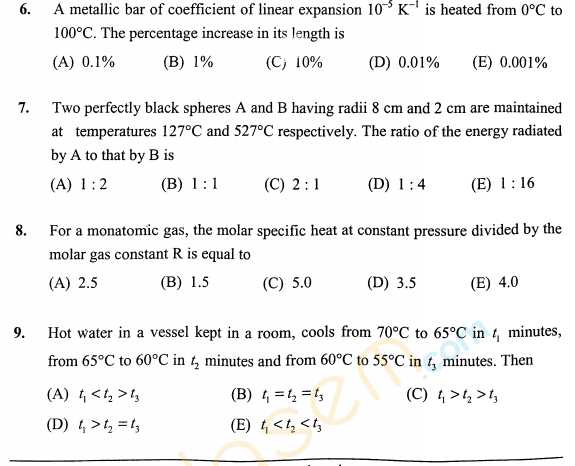  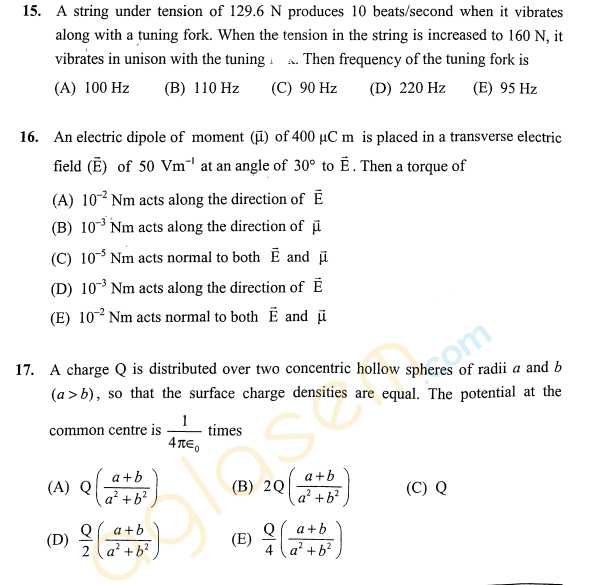 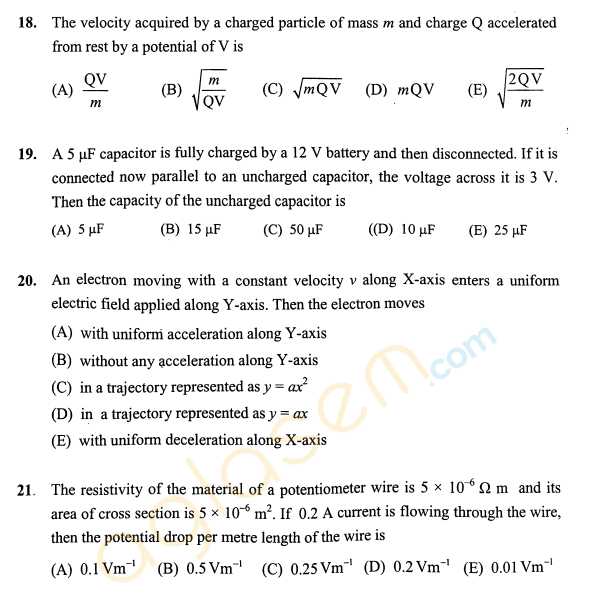 For more details, you can refer to the attached file |