|
#2
27th September 2016, 01:18 PM
| |||
| |||
| Re: IIT JEE Test Series
As you want I am here giving you sample test paper for IIT JEE exam. Which ordering of compounds is according to the decreasing order of the oxidation state of nitrogen? (A) HNO3, NO, NH4Cl, N2 (B) HNO3, NO, N2, NH4Cl (C) HNO3, NH4Cl, NO, N2 (D) NO, HNO3, NH4Cl, N2 Ans. (B) In allene (C3H4), the type(s) of hybridisation of the carbon atoms is (are) : (A) sp and sp3 (B) sp and sp2 (C) only sp3 (D) sp2 and sp3 Ans. (B) As per IUPAC nomenclature, the name of the complex [Co(H2O)4(NH3)2]Cl3 is : (A) Tetraaquadiaminecobalt (III) chloride (B) Tetraaquadiamminecobalt (III) chloride (C) Diaminetetraaquacoblat (III) chloride (D) Diamminetetraaquacobalt (III) chloride Ans. (D) Sol. [Co(H2O)4 (NH3)2 ]Cl3 The colour of light absorbed by an aqueous solution of CuSO4 is : (A) organge-red (B) blue-green (C) yellow (D) violet Ans. (A) Sol. CuSO4 will be absorbing orange-red colour & hence will be of blue colour. Choose the correct reason(s) for the stability of the lyophobic colloidal particles. (A) Preferential adsorption of ions on their surface from the solution. (B) Preferential adsorption of solvent on their surface from the solution. (C) Attraction between different particles having opposite charges on their surface. (D) Potential difference between the fixed layer and the diffused layer of opposite charges around the colloidal particles. Ans. (AD) IIT JEE exam sample paper 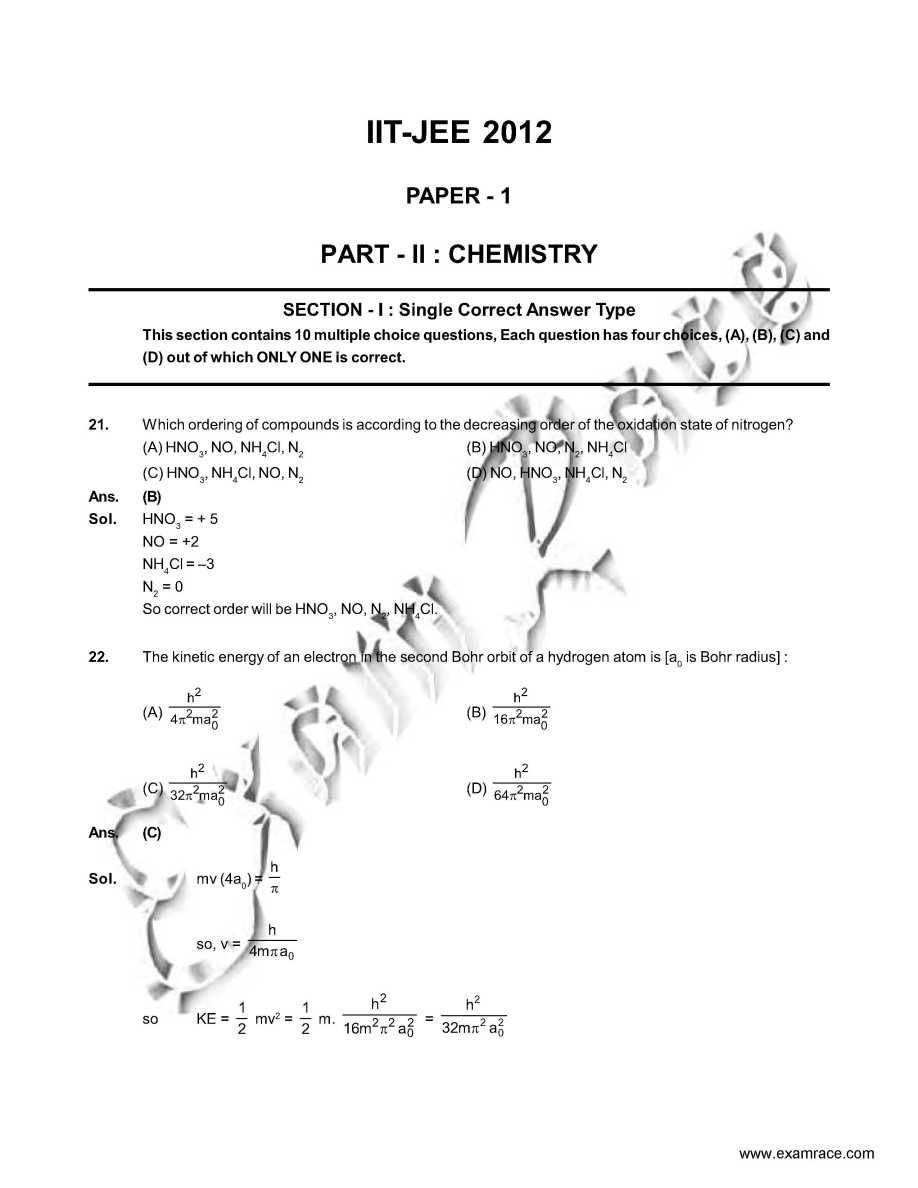 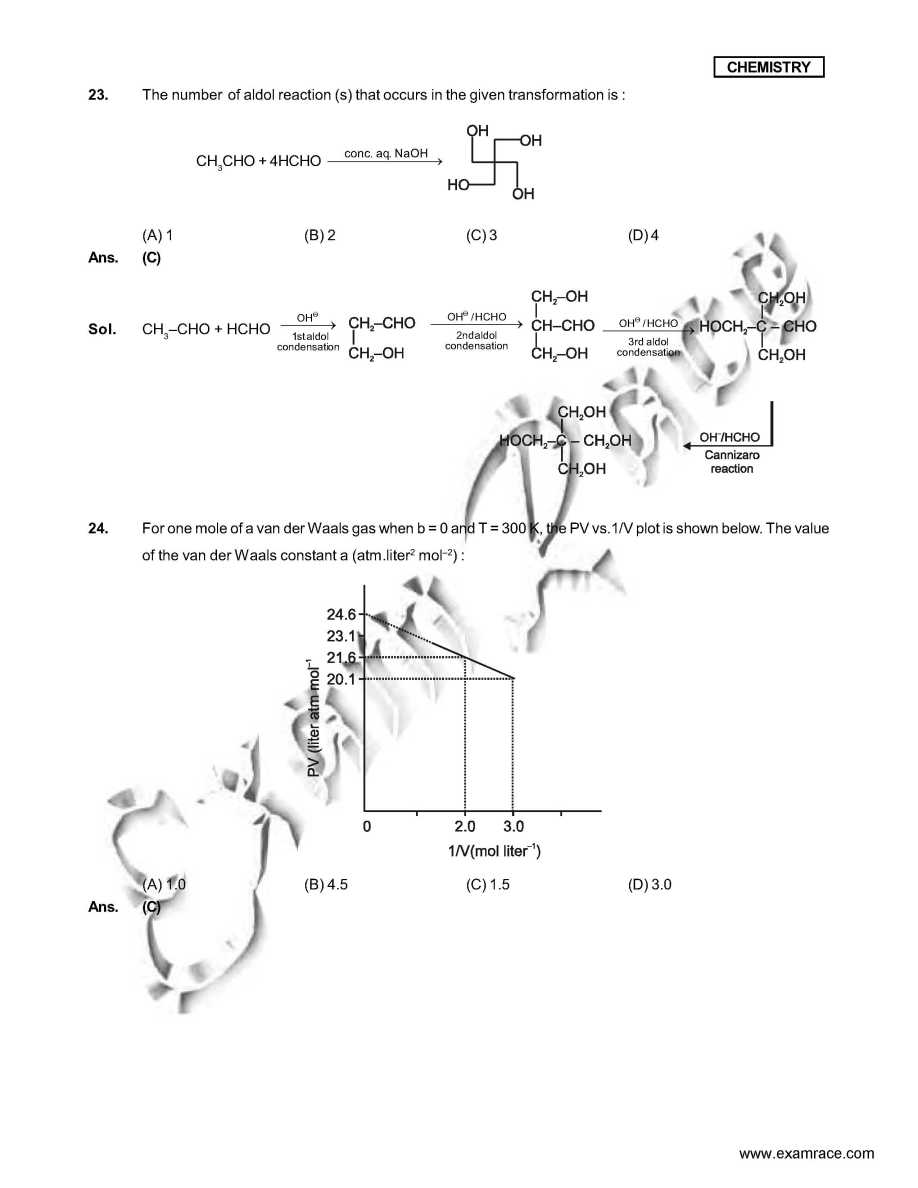 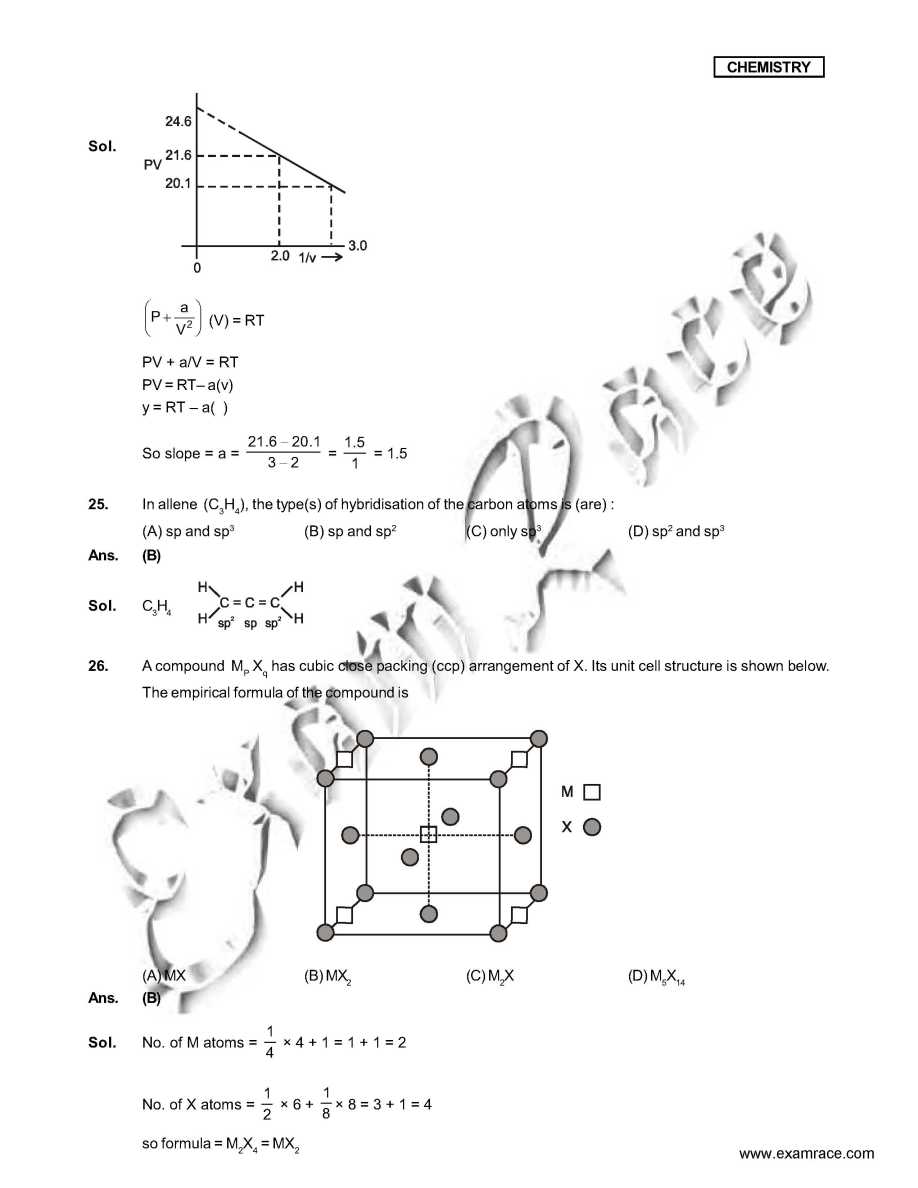 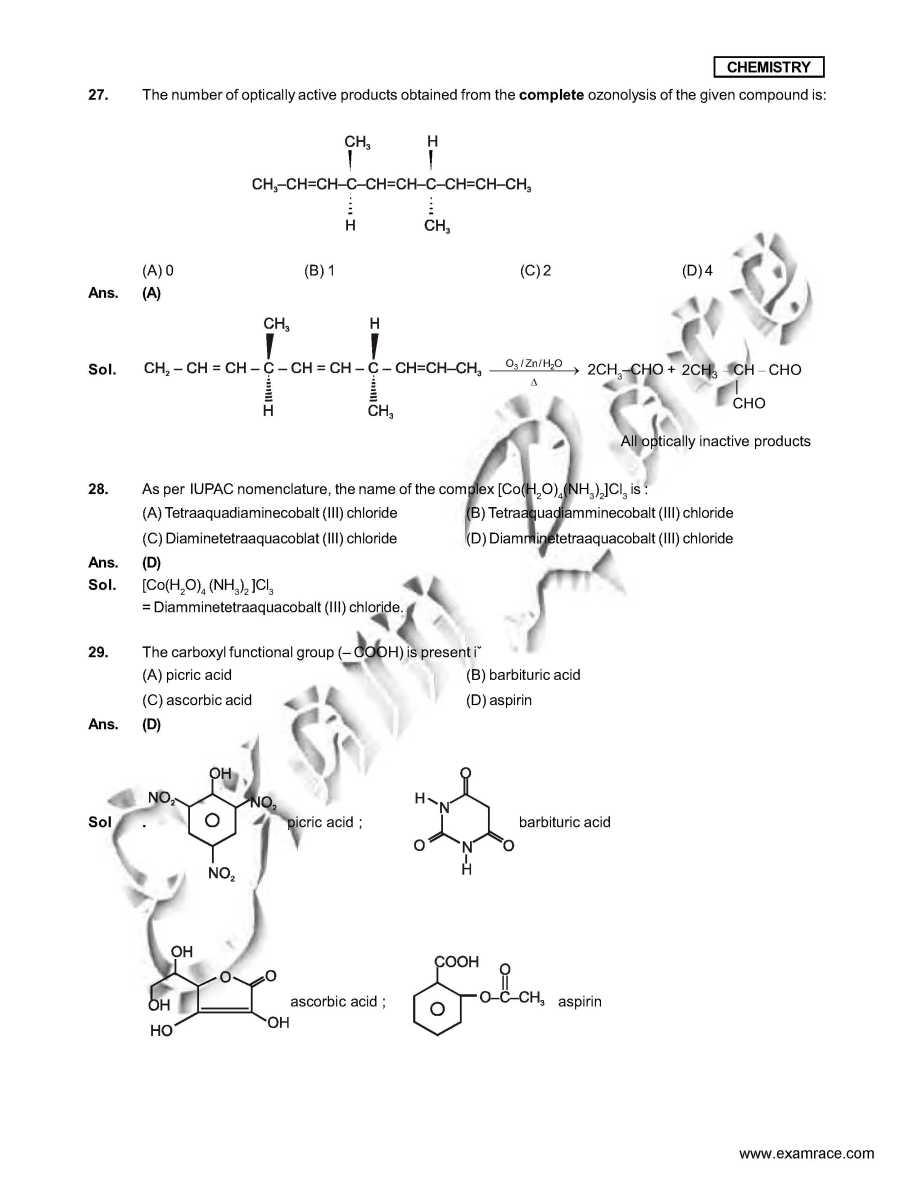 Here is the attachment . |