|
#2
5th March 2016, 07:55 AM
| |||
| |||
| Re: Concentrated Nitric Acid
Nitric corrosive is made by response of nitrogen dioxide (NO2) with water. Ordinarily, the nitric oxide created by the response is reoxidized by the oxygen in air to deliver extra nitrogen dioxide. Percolating nitrogen dioxide through hydrogen peroxide can enhance corrosive yield. At the point when the arrangement contains more than 86% HNO3, it is alluded to as smouldering nitric corrosive. Contingent upon the measure of nitrogen dioxide present, smouldering nitric corrosive is further portrayed as white raging nitric corrosive or red seething nitric corrosive, at fixations above 95%. The concoction recipe of nitric corrosive is HNO3, as it is comprised of one hydrogen particle, one nitrogen molecule, and three oxygen iotas. Its substance structure demonstrates that it is a planar particle and it has two reverberation shapes. 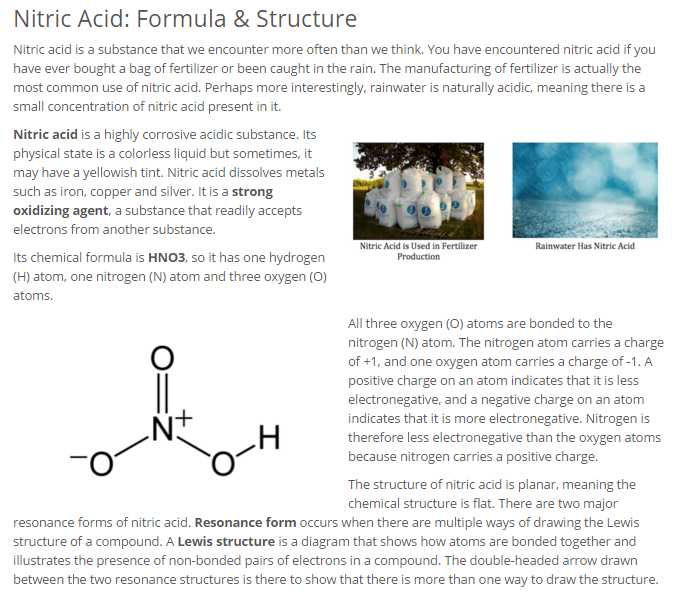 Nitric corrosive is a substance that one experience more frequently than one might suspect. One have had experienced nitric corrosive in the event that one has ever purchased a pack of compost or been gotten in the downpour. The assembling of manure is really the most well-known utilization of nitric corrosive. Maybe all the more interestingly, water is normally acidic, which means there is a little convergence of nitric corrosive present in it. Uses of Nitric Acid: Fertilizer Aerospace Engineering Nitric acid is also used as precursor to make organic nitrogen-containing compounds, such as nylon.  |