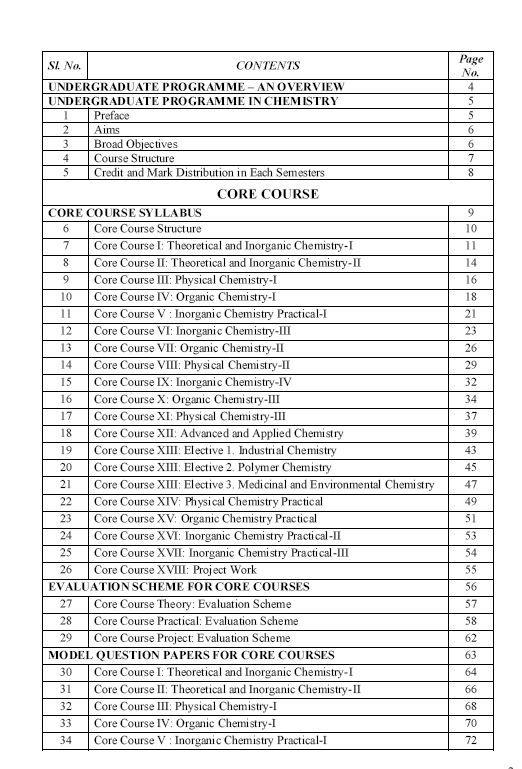
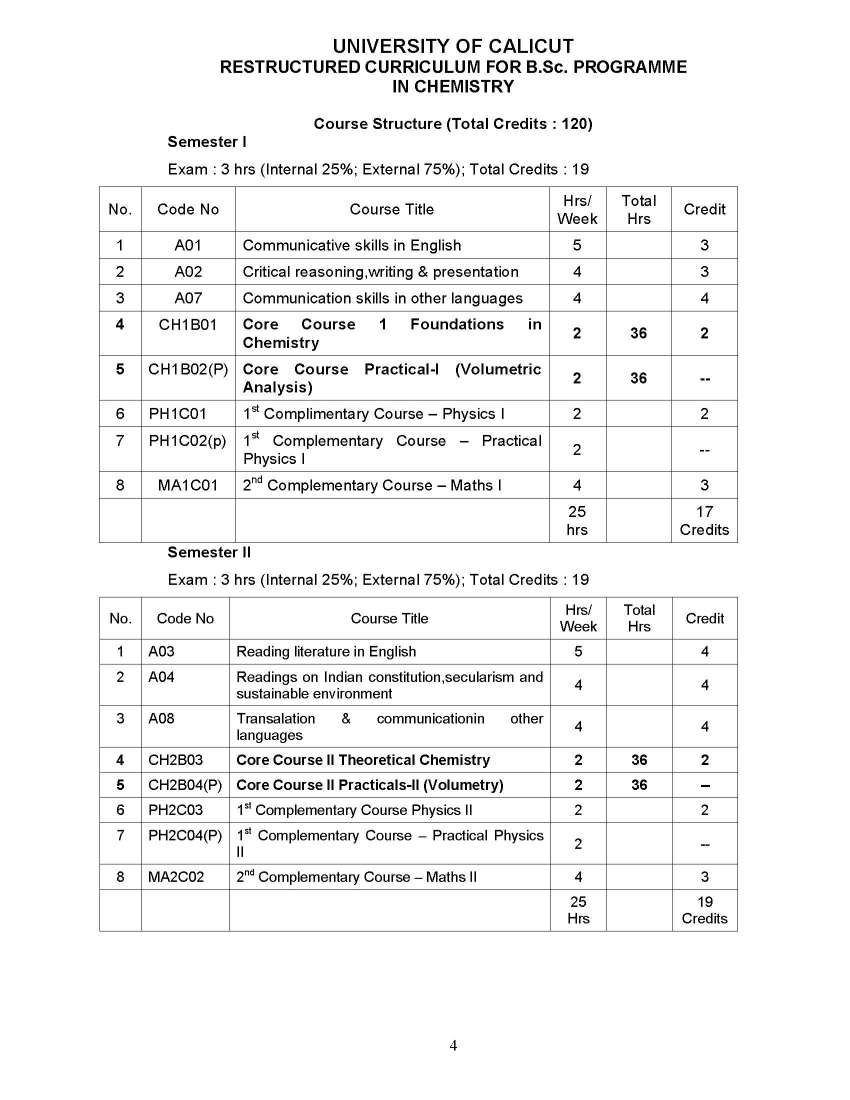
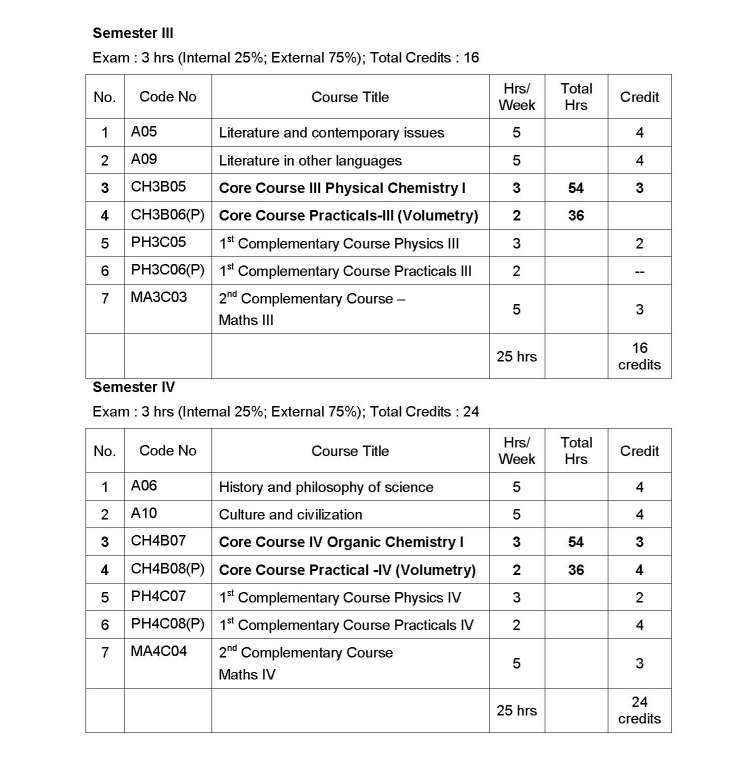
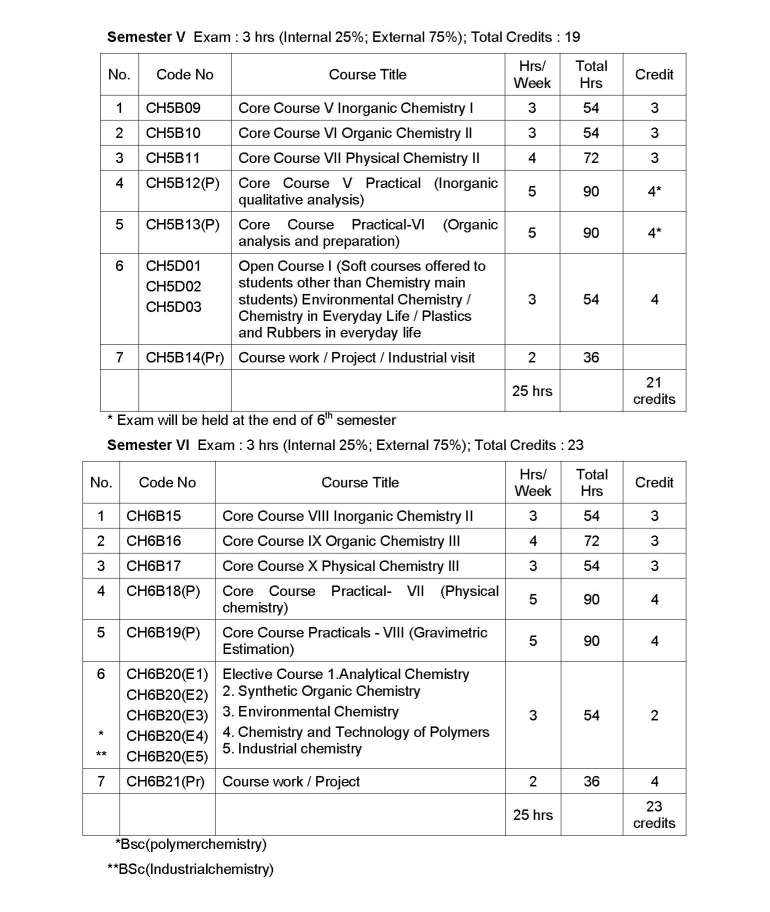
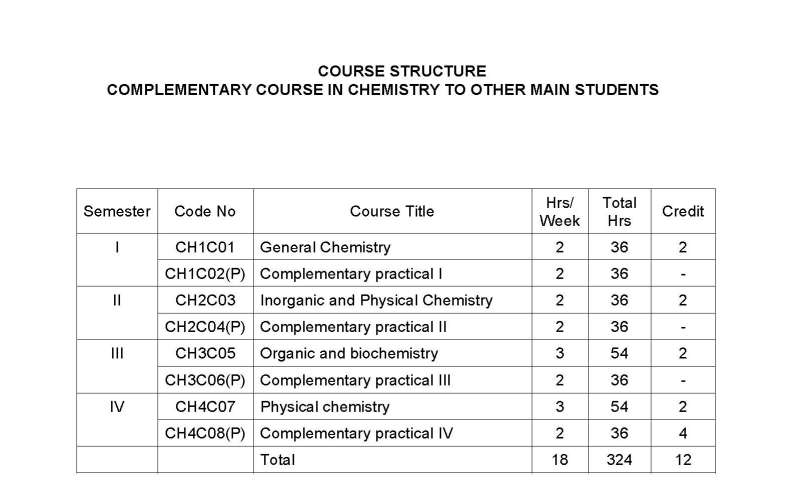
As you are asking for Calicut University B.Sc Chemistry program Syllabus , so on your demand I am providing same here :
SEMESTER I
Course Code: CHE1B01
Core Course I: THEORETICAL AND INORGANIC CHEMISTRY - I
Total Hours: 36; Credits: 2; Hours/Week: 2
Module I: Chemistry as a Discipline of Science (6 hrs)
What is Science? - Scientific statements - Scientific methods Observation - Posing a question -
Formulation of hypothesis Experiment Theory Law - Revision of scientific theories and laws - Role
of concepts and models in science - Scientific revolution.
Evolution of chemistry - Ancient speculations on the nature of matter - Early form of chemistry
Alchemy - Origin of modern chemistry - Branches of chemistry -Interdisciplinary areas involving
physics and biology.
Objectives of Chemical Research - Research design. Components of a research project: Introduction,
review of literature, scope, materials and methods, results and discussion, conclusions and
bibliography.
Module II: Some Basic Chemical Concepts (3 hrs)
Symbol of elements Atomic number and mass number - Atomic mass Isotopes, isobars and isotones -
Molecular mass - Mole concept Molar volume - Oxidation and reduction Oxidation number and
valency Variable valency - Equivalent mass.
Methods of expressing concentration: Weight percentage, molality, molarity, normality, mole fraction,
ppm and millimoles.
Module III: Analytical Chemistry - I (9 hrs)
Laboratory Hygiene and Safety: Storage and handling of chemicals. Simple first aids: Electric shocks,
fire, cut by glass and inhalation of poisonous gases - Accidents due to acids and alkalies - Burns due to
phenol and bromine. Disposal of sodium and broken mercury thermometer - Use of calcium chloride and
silica gel in desiccators. Awareness of Material Safety Data Sheet (MSDS) R & S Phrases (elementary
idea only) Safe laboratory practices Lab safety signs.
Volumetric Analysis: Introduction - Primary and secondary standards Standard solutions - Theory of
titrations involving acids and bases, KMnO4, K2Cr2O7, I2 and liberated I2 - Complexometric titrations.
Indicators: Theory of acid-base, redox, adsorption and complexometric indicators. Double burette
method of titration: Principle and advantages.
Significant figures Comparison of results.
Module IV: Atomic Structure (9 hrs)
Introduction based on historical development John Dalton's atomic theory Thomsons atom model
and its limitations Rutherfords atom model and its limitations - Failure of classical physics Black
body radiation - Plancks quantum hypothesis - Photoelectric effect - Generalization of quantum theory
Atomic spectra of hydrogen and hydrogen like atoms - Ritz-combination principle Bohr theory of
atom Calculation of Bohr radius, velocity and energy of an electron - Explanation of atomic
spectra Rydberg equation - Limitations of Bohr theory - Sommerfeld modification - Louis de Broglie's
matter waves Wave-particle duality - Electron diffraction - Heisenberg's uncertainty principle.
Module V: Nuclear Chemistry (9 hrs)
Natural radioactivity Modes of decay Group displacement law Theories of disintegration Rate of
decay Decay constant Half life period Gieger-Nuttall rule Radioactive equilibrium
Disintegration series Transmutation reactions using protons, deutrons, α-particles and neutrons
Artificial radioactivity Positron emission and K electron capture Synthetic elements.
Nuclear stability N/P ratio Packing fraction Mass defect Binding energy Nuclear forces
Exchange theory and nuclear fluid theory Nuclear fission - Atom bomb Nuclear fusion Hydrogen
bomb - Nuclear reactors - Nuclear reactors in India.
Isotopes: Detection Aston's mass spectrograph Separation of isotopes by gaseous diffusion method
and thermal diffusion method Application of radioactive isotopes
14C dating Rock dating Isotopes
as tracers Study of reaction mechanism (ester hydrolysis) Radio diagnosis and radiotherapy.
Calicut University B.Sc Chemistry program Syllabus